As it emerges from some studies presented across different cancers, toxicity remains a major challenge
Ever since KRAS became a ‘druggable’ oncogenic driver, the number of selective inhibitors has been growing steadily, with a current focus on G12C and G12D mutations. However, comparable or superior efficacy to other targeted treatments has not yet been demonstrated, and the toxicity of KRAS inhibitors continues to represent a major challenge, as phase I findings presented at the ESMO Congress 2025 (Berlin, 17–21 October) highlight.
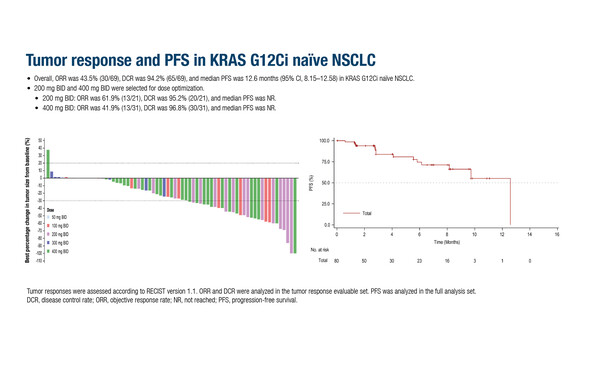
Among efficacy-evaluable patients with KRAS G12C-mutant advanced solid tumours who had progressed on, or were not eligible for, standard treatment, the KRAS G12C inhibitor HRS-7058 achieved objective response rates (ORRs) of 43.5% in 69 KRAS G12C inhibitor-naïve patients with non-small cell lung cancer (NSCLC), 20.6% in 34 KRAS G12C inhibitor-pre-treated patients with NSCLC, 34.1% in 41 patients with colorectal cancer (CRC) and 75.0% in patients with pancreatic ductal adenocarcinoma (PDAC) (Abstract 914O, key results in the box below). “The efficacy appears similar to existing KRAS inhibitors,” says Prof. Christophe Massard from Université Paris Saclay, Gustave Roussy, France. The data are consistent with other studies of KRAS G12C inhibitors that have failed to demonstrate robust efficacy due to numerous resistance mechanisms, including loss of tumour suppressor gene functions and activation of bypass signal transduction pathways (Genes Dev. 2025;39:132‒162). Notably, HRS-7058 showed encouraging activity in patients with NSCLC who had previously been treated with a KRAS G12C inhibitor. “This could provide insights into the distinct mechanisms of resistance to KRAS G12C inhibitors,” he adds. The safety profile of HRS-7058 was manageable with no dose-limiting toxicities (DLTs).
Some unexpected toxicities were observed with HRS-4642, a highly selective, non-covalent KRAS G12D inhibitor, which was investigated in 84 patients with advanced KRAS G12D-mutant solid tumours, all of whom had received prior chemotherapy, immunotherapy or targeted therapy (Abstract 915O, key results in the box below). While no DLTs were observed during dose escalation and the maximum tolerated dose was not reached, grade ≥3 TRAEs occurred in 23.8% of patients, most commonly hypertriglyceridaemia, neutropenia and hypercholesterolaemia. One patient discontinued treatment due to a TRAE, and 1 treatment-related death was reported. “Although the safety profile was mostly acceptable, unexpected toxicities deserve further study,” notes Massard. This agent has a different mechanism of action to ASP3082, a KRAS G12D selective protein degrader presented previously (Ann Oncol. 2024;35(Suppl 2):S486–487). ASP3082 harnesses the body’s natural protein waste disposal process (the ubiquitin-proteasome system) to break down and eliminate the KRAS G12D protein to inhibit KRAS downstream signalling. He adds, “Phase I data suggest that this novel mechanism of action may be associated with less toxicity than KRAS G12D inhibitors; only 5% of patients experienced grade ≥3 TRAEs with ASP3082.”
As presented at a Proffered paper session in Berlin, HRS-4642 showed early activity in lung and pancreatic cancers, similar to another novel, non-covalent KRAS G12D inhibitor, INCB161734. The latter was evaluated as monotherapy in an ongoing phase I first-in-human study in 138 patients with advanced or metastatic KRAS G12D-mutant solid tumours (83 with PDAC, 46 with CRC, 3 with NSCLC, 2 with ovarian cancer and 4 with other tumour types), also presented at the ESMO Congress (Abstract 916O, key results in the box below). Similar ORRs were reported with HRS-4642 and INCB161734 in patients with PDAC.
An early molecular response (≥90% reduction in KRAS G12D variant allele frequency) in patients with detectable baseline circulating tumour DNA (ctDNA) was seen in 41% and 72% of patients treated with 600 mg or 1200 mg INCB161734, respectively. “It is interesting to note that in the study investigating INCB161734, early serial ctDNA assessments were implemented to inform dose escalation. The inclusion of ctDNA analysis could offer a useful surrogate marker of response,” adds Massard. The safety profile of INCB161734 was manageable with no dose-limiting toxicities or treatment discontinuations due to TRAEs.
Concluding, Massard notes that these are very early-phase data and further evidence is needed before firm conclusions can be drawn. Future research should prioritise combination strategies, particularly with PD-1 inhibitors and chemotherapy, to overcome resistance. A second part of the INCB161734 study is currently enrolling and will test the KRAS G12D inhibitor in combination with standard therapies for CRC and PDAC. It is hoped that combination strategies will lead to improved treatment responses and durability of response.
Huang D, et al. HRS-7058, a KRAS G12C inhibitor (G12Ci), in advanced solid tumors with KRAS G12C mutation: A phase 1, multi-center, first-in-human study. ESMO Congress 2025 - Abstract 914O
- KRAS G12Ci-naïve NSCLC (n=69): ORR 43.5%, DCR 94.2%
- KRAS G12Ci-pre-treated NSCLC (n=34): ORR 20.6%, DCR 91.2%
- CRC (n=41): ORR 34.1%, DCR 78.0%
- PDAC (n=4): ORR 75.0%, DCR 100%
- Grade ≥3 TRAEs in 14.1% pts
Zhou C, et al. KRAS G12D inhibitor HRS-4642 in patients with KRAS G12D-mutant advanced solid tumors: A phase 1 trial. ESMO Congress 2025 - Abstract 915O
- ORR: 23.7 % in NSCLC, 20.8% in PDAC
- DCR: 76.3% in NSCLC, 79.2% in PDAC
- mPFS in NSCLC: 6.3 months (95% CI 1.1‒11.0) with 800 mg Q2W and 8.4 months (95% CI 1.4‒NR) with 1200 mg Q2W
- mPFS in PDAC: 4.4 months (95% CI 2.6‒7.2) with 1200 mg Q2W
- mOS: in NSCLC: 7.5 months (95% CI 4.1‒NR) with 800 mg Q2W and NR (95% CI 8.4‒NR) with 1200 mg Q2W
- mOS in PDAC: NR (95% CI 4.2‒NR) with 1200 mg Q2W
- Grade ≥3 TRAEs in 23.8% pts
Desai J, et al. Preliminary phase 1 results of INCB161734, a novel oral KRAS G12D inhibitor, in patients with advanced or metastatic solid tumors. ESMO Congress 2025 - Abstract 916O
- At 600 mg qd in patients with PDAC (n=25)
- ORR: 20% (5 pts)
- DCR (PR + stable disease): 64% (16 pts)
- ORR: 34% (10 pts)
- DCR: 86% (25 pts)
- TRAEs in 15% of pts: nausea (58%), diarrhoea (51%), vomiting (46%) and fatigue (18%)