Phase I–II studies showcase the clinical potential of novel ADCs with enhanced targeting and/or payload strategies
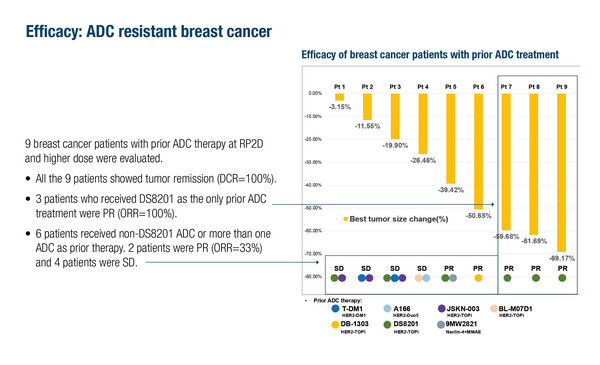
We are witnessing a major explosion in the development of antibody–drug conjugates (ADCs) . While HER2 remains the most frequent ADC target, several trials presented at the ESMO Asia Congress 2025 (Singapore, 5–7 December) indicate benefits with targets beyond HER2 and payloads other than topoisomerase I inhibitors, which are being explored to minimise cross-resistance. In a phase I–Ib study in 30 heavily pre-treated cancers, the HER2-targeting eribulin-based ADC, SMP-656, led to partial responses (PRs) in 4 ADC-naïve patients with breast cancer and high HER2 expression and 1 of 2 patients with low HER2 expression (Abstract 89MO, key results in the table below). In addition, all 9 patients with breast cancer who received prior ADC treatment showed tumour remission: PRs were reported in all 3 who had received the HER2/topoisomerase I inhibitor ADC, trastuzumab deruxtecan (T-DXd; formerly DS8201), and in 2 of 6 patients who had received a non-T-DXd ADC or more than 1 prior ADC. A total of 23.3% of patients developed grade ≥3 treatment-emergent adverse events (AEs), none of which led to study discontinuation.
“There is currently no approved ADC with the tubulin inhibitor, eribulin, as the payload,” notes Dr Paolo Tarantino from the Dana-Farber Cancer Institute and Harvard Medical School in Boston, MA, USA. “These promising initial data, particularly the evidence of tumour activity in patients who had received prior ADCs, suggest that this type of agent may be especially useful in helping to provide an effective non-cross-resistant option to treat tumours resistant to topoisomerase I inhibitor-based ADCs.”
A phase II trial of BL-M07D1 – another HER2-targeting ADC but one that is linked to a novel topoisomerase I inhibitor (Ed-04) – reported a confirmed objective response rate (ORR) of 56.9% among 58 patients with previously treated HER2-mutated non-small cell lung cancer (Abstract 160MO, key results in the table below). At a median follow-up of 10.3 months, the median progression-free survival (PFS) was not reached and the 6-month PFS rate was 88.7%. Grade ≥3 treatment-related AEs, mainly haematological, led to a discontinuation rate of 3.4%. There was one death assessed by the investigator as possibly related to BL-M07D1. A phase III registrational trial, comparing BL-M07D1 with pembrolizumab and platinum in the first-line treatment of HER2-mutated advanced or metastatic NSCLC, has recently started (NCT07178795).
“With its, albeit novel, topoisomerase I inhibitor payload, the BL-M07D1 ADC is similar to T-DXd and the ORR was in line with what might be expected with the established ADC in this scenario,” observes Tarantino. “The 6-month PFS rate is impressive, and it will be interesting to see if results will be confirmed in a larger study.” In terms of toxicity, the safety profiles of SMP-656 or BL-M07D1 are reassuring, particularly the fact that both studies reported no interstitial lung disease.
Two other trials, both in gynaecological cancers, investigated ADCs carrying topoisomerase I inhibitor payloads, but each using immune checkpoint molecules as ADC targets. In a phase II study in 28 efficacy-evaluable patients with previously treated recurrent/metastatic cervical cancer, the anti-PD-L1 ADC, HLX43, led to an ORR of 42.9% (Abstract 602O, key results in the table below). Among 10 patients receiving the highest dose of 3 mg/kg, the ORR was 70.0%. A total of 70% of treatment-emergent AEs were grade ≥3, which were mostly haematological. Some patients had immune-related AEs, which Tarantino suggests shows that targeting PD-L1 in this context not only ensures delivery of the payload but may also enhance the immune response.
The final study reported that a B7-H3 protein-targeting ADC, DB-1311/BNT324, led to confirmed ORRs of 33.3% among 30 efficacy-evaluable patients with previously treated advanced/recurrent cervical cancer and 58.3% among 12 patients with platinum-resistant ovarian cancer (Abstract 603O, key results in the table below). Corresponding median PFS durations were 7.0 months and 8.2 months, respectively. Grade ≥3 treatment-related AEs occurred in 62.8% of patients.
“We know that ADCs can be effective in some gynaecological cancers. However, there is currently no topoisomerase I inhibitor ADC with regulatory approval for cervical or ovarian cancer, with the exception of T-DXd for selected patients with HER2-positive disease,” explains Tarantino. “The results presented on HLX43 and DB-1311/BNT324 show the real promise of topoisomerase I inhibitor ADCs in both of these tumour types.”
Tarantino concludes: “Around half of the ADCs being developed at this time are emerging from China. As we move forward, increased global collaboration will help maximise the potential for innovative therapies to benefit patients around the world.”
At a glance:
Zhang J, et al. Phase I/Ib data of SMP-656: A novel eribulin-based HER2 ADC in solid tumor. ESMO Asia Congress 2025 - Abstract 89MO
- HER2-expressing or mutated solid tumours (N=30; breast cancer n=25)
- ORR (all PRs) ADC naïve: 6/6
- ORR (all PRs) prior ADC: 5/6
- Grade ≥3 TEAEs: 23.3%
Zhang L, et al. BL-M07D1, a novel anti-HER2 antibody-drug conjugate (ADC), in subjects with metastatic HER2-mutant non-small cell lung cancer (NSCLC). ESMO Asia Congress 2025 - Abstract 160MO
- Previously treated HER2-mutated NSCLC (N=58)
- cORR: 56.9%
- Median PFS: NR; 6-month PFS rate: 88.7%
- Grade 3–4 TRAEs: anaemia (32.8%), thrombocytopenia (39.7%), leukopenia (36.2%) and neutropenia (37.9%)
Yu J, et al. Efficacy and safety of HLX43 (an anti-PD-L1 ADC) in recurrent/metastatic cervical cancer (CC): A randomised, multicentre, phase II study. ESMO Asia Congress 2025 - Abstract 602O
- Previously treated recurrent/metastatic cervical cancer (N=30)
- ORR across doses (n=28): 42.9%
- Highest dose of 3 mg/kg (n=10): 70.0%
- Grade ≥3 TEAEs: 70.0%
Chang C-L, et al. DB-1311/BNT324 (a novel B7H3 ADC) in patients with advanced cervical cancer or platinum-resistant recurrent ovarian cancer. ESMO Asia Congress 2025 - Abstract 603O
- Cohort of patients from phase I–II study (N=43)
- Cervical cancer (n=31, efficacy-evaluable n=30):
- cORR: 33.3%; mPFS: 7.0 months
- PROC (n=12):
- cORR: 58.3%; mPFS: 8.2 months
- Grade ≥3 TRAEs: 62.8%