Initial results of a phase II trial with rezatapopt suggest real promise in an area that has eluded druggable targeting
“Despite it being one of the most frequently mutated genes in human tumours, efforts over the past 20 years to target the TP53 mutation have been in vain and there is still no effective agent available in the clinic. Targeting MDM2, a downstream effector, is the closest we have got,” explains Prof. Toshio Shimizu from Kansai Medical University Hospital, NEXT Oncology KMU JAPAN, Osaka, Japan, warmly welcoming the encouraging findings from the global, basket, phase II PYNNACLE trial presented at the ESMO Asia Congress 2025 (Singapore, 5–7 December). “The benefits from monotherapy with rezatapopt – a novel, oral, small molecule p53 reactivator targeting the TP53 Y220C mutation – in heavily pre-treated patients finally provide hope for targeting other TP53 mutations,” he added.
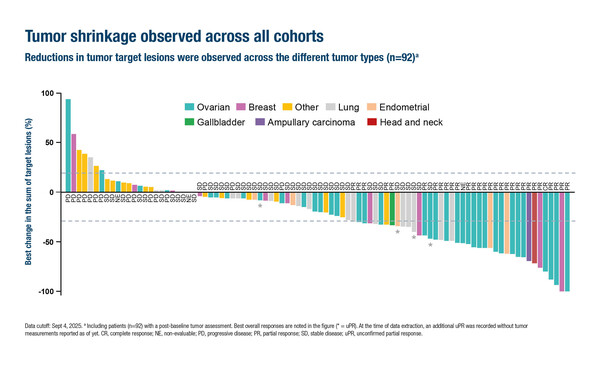
The TP53 Y220C mutation is found in around 1% of solid tumours, with higher levels of expression in ovarian cancer (Ann Oncol. 2024;35[Suppl 4]:S1581). Rezatapopt led to an investigator-assessed objective response rate (ORR) of 34.0% among 103 efficacy-evaluable patients with heavily pre-treated solid tumours expressing a TP53 Y220C mutation, but without a KRAS single nucleotide variant (LBA2). An ORR of 45.8% was reported among 48 patients with ovarian cancer. Tumour shrinkage was demonstrated across all tumour types enrolled.
The median time to response was 1.3 months in both the total cohort and the ovarian cancer cohort and the median durations of response were 7.6 months and 8.0 months, respectively. At the data cut-off, 40% of patients remained on treatment. Among 78 patients with baseline and on-treatment circulating tumour DNA available, all those responding to rezatapopt showed a reduction in TP53 Y220C variant allele frequency. Results for the primary endpoint of ORR by blinded independent central review are still awaited.
The most common grade ≥3 treatment-related adverse events (TRAEs) were increases in aspartate aminotransferase and alanine aminotransferase levels (6% each) and anaemia, platelet count decrease and maculo-papular rash (4% each). Four patients discontinued treatment due to TRAEs. “It is reassuring that adverse events were generally manageable,” observes Shimizu, “And building on the phase I findings (Mol Cancer Ther. 2023;22[Suppl 12]:LBA25), the administration of rezatapopt with food markedly reduced the incidence of gastrointestinal adverse events like nausea and vomiting.”
Given the likely extensive clinical gains of targeting TP53 mutations, this continues to be an intense area of research. “A wide variety of approaches are being investigated, including MDM2 inhibitors, gene therapy with p53-expressing adenoviruses and p53-targeted antibody-based treatment, but most compounds are at a very early stage of development. Against this background, these initial results with rezatapopt give hope for wider clinical potential,” concludes Shimizu.
Programme details:
Tan DS, et al. Rezatapopt for advanced solid tumours with a TP53 Y220C mutation: Initial analysis of the PYNNACLE phase II study. ESMO Asia Congress 2025 - LBA2