Further studies are needed to understand the position of the fourth-generation agents NVL-520 and NVL-655 in the treatment paradigm of mutated lung cancer
Encouraging efficacy results from early-phase studies of two novel tyrosine kinase inhibitors (TKIs) in ALK mutation-positive non-small cell lung cancer (NSCLC) presented at the ESMO Congress 2024 (Barcelona, 13–17 September) hint at promising new therapies for patients who have exhausted existing treatment options.
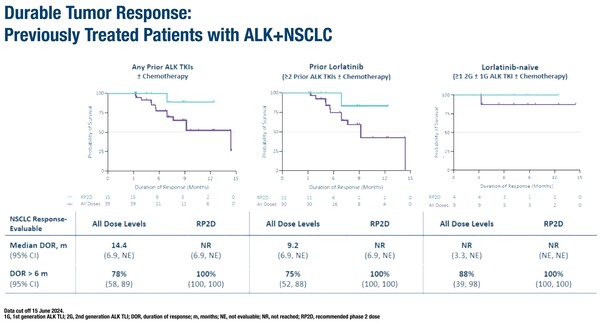
In the dose-finding phase I ALKOVE-1 study, the brain-penetrant, ALK-selective TKI NVL-655 was investigated in 133 patients with heavily pre-treated solid tumours, primarily NSCLC (Abstract 1253O). Preclinical studies showed that NVL-655 is sensitive to the acquired ALK mutation G1202R, which occurs after treatment with second- and third-generation ALK TKIs (Mol Cancer Ther. 2021;20(12_Suppl.):Abstract P244).
In the presented study, the overall objective response rate (ORR) with NVL-655 was 38%, 35% in those who had received ≥2 prior ALK-targeting TKIs (including second-generation TKIs and lorlatinib) and 53% in lorlatinib-naïve patients. Overall, the duration of response was 14.4 months, with 9.2 months in the heavily pre-treated group, and 14.4 months in those with ALK G1202R mutations, regardless of prior lorlatinib treatment, while it was not reached in the lorlatinib-naïve group. The overall ORR in patients receiving the recommended phase II dose of NVL-655 150 mg once daily was 38%, with complete resolution of CNS metastases in some lorlatinib-experienced patients. The most common treatment-related adverse events were elevation of liver enzymes, constipation, dysgeusia and nausea.
Commenting on the study findings, Prof. Tony Mok from the Chinese University of Hong Kong, Hong Kong SAR China, says: “While the ORR and duration of response results are reasonable for NVL-655, the recent 5-year results from the CROWN study of lorlatinib versus crizotinib in treatment-naïve, advanced ALK-positive NSCLC, showing progression-free survival of more than 60 months with lorlatinib (J Clin Oncol. 2024;42(Suppl. 17):LBA8503), will have a tremendous impact on the NSCLC treatment paradigm, with more likely use of lorlatinib in the first line rather than as subsequent line of therapy. NVL-655, a fourth-generation TKI, could serve as an additional treatment option with durable response for patients who have failed on lorlatinib.”
Also presented at the Congress, the phase 1/2 ARROS-1 study showed that the novel ROS1 fusion-positive-targeting zidesamtinib (NVL-520) was associated with an ORR of 44% in the 71 evaluable patients of a total of 104 patients with solid tumours (99 with NSCLC) and diverse ROS1 fusion and resistance mutations who had received prior ROS1-targeting TKIs with or without chemotherapy (Abstract 1256MO). A duration of response of >12 months was achieved by 67% of patients overall, and by 71% of patients who were naïve to repotrectinib. Commenting on the results, Mok says, “NVL-520 appears to have good efficacy in this setting, but the results fall short of those for the ROS1-selective TKI taletrectinib in the TRUST-I study, which was recently associated with an ORR of 91% in TKI-naïve patients and 52% in TKI pre-treated patients (J Clin Oncol. 2024;42(Suppl. 16):Abstract 8520). There is a lack of comparative trials for ROS1-mutant-positive NSCLC to show the optimal sequence of treatment.”
Mok thinks there are two key areas that need to be explored in the NSCLC setting. “Similar to what was done in the ALINA trial (N Engl J Med. 2024;390:1265–1276), where adjuvant alectinib was found to be superior to adjuvant platinum-based chemotherapy in early-stage NSCLC, we need to know whether these newer TKIs could have efficacy in the early-stage, adjuvant setting. Secondly, first-line agents targeting these relatively rare tumour mutations have good response durations, but treatment failure is inevitable. There is an unmet need for effective second-line agents to offer these patients after first-line failure,” he concludes.
Programme details
Drilon AE, et al. Phase 1/2 ALKOVE-1 study of NVL-655 in ALK-positive (ALK+) solid tumors. ESMO Congress 2024, Abstract 1253O
Proffered Paper Session – NSCLC metastatic, 14.09.2024, h. 08:30 – 10:00, Barcelona Auditorium – Hall 2
Besse B, et al. Phase 1/2 ARROS-1 study of zidesamtinib (NVL-520) in ROS1 fusion-positive solid tumors. ESMO Congress 2024, Abstract 1256MO
Mini Oral Session – NSCLC metastatic, 14.09.2024, h. 10:15 – 11:45, Santander Auditorium – Hall 5