Efficacy and safety of telisotuzumab adizutecan was investigated in patients with EGFR wild type non-squamous disease
An antitumour activity of telisotuzumab adizutecan (ABBV-400), a c-Met directed antibody-drug conjugate (ADC), was observed in previously treated patients with EGFR wild type (WT) non-squamous non-small cell lung cancer (NSCLC), according to preliminary data presented at the ESMO Congress 2024, held in Barcelona, Spain, in September.
ABBV-400 is being investigated in adults with advanced solid tumors in an ongoing phase I study (NCT05029882), and in dose escalation showed a manageable safety profile and antitumor activity (J Clin Oncol. 2023;41(16 suppl):3015). The EGFR WT cohort involved 48 patients with EGFR WT non-squamous NSCLC whose disease had progressed on prior platinum-based chemotherapy and an immune checkpoint inhibitor and/or targeted therapy.
Antitumor activity was observed with ABBV-400 when dosed at 2.4 and 3.0 mg/kg administered once every 3 weeks (n=39 and 9, respectively), with an overall response rate (ORR) of 47.9% and clinical benefit rate of 85.4%. With a median 6.8 months of follow-up, 17 patients remain on treatment, and duration of response was immature at data cutoff.
Toxicity management represents a major challenge for the use of ADCs in NSCLC. The safety profile of ABBV-400 was generally consistent with that observed in the total study population (n=367). Most treatment-emergent adverse events (TEAEs) were hematological (any grade, 69%; G 3–4, 35%) and gastrointestinal (any grade, 65%; G 3–4, 4%). Eight (17%) patients had fatal TEAEs, 3 of which were disease progression and the remaining were infection (6%), respiratory distress (2%), and pneumonitis (2%).
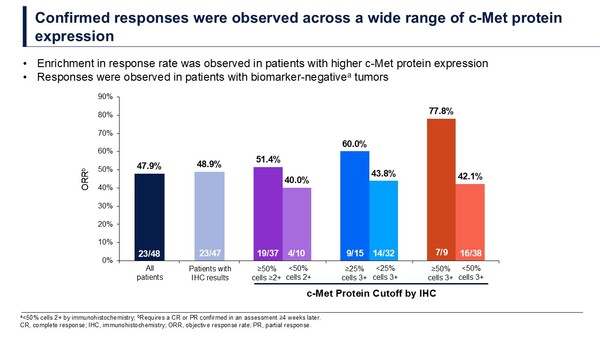
Data presented at the Congress also reported an enrichment in response rate in patients with higher c-Met protein expression, and responses were observed in patients with biomarker-negative tumors (Figure). However, further investigation is ongoing to establish the association between c-Met protein expression and treatment response.
Abstract discussed at the ESMO Congress 2024
de Miguel M, et al. ABBV-400, a c-Met Protein–Targeting Antibody-Drug Conjugate, in Patients With Advanced EGFR Wildtype Non-Squamous Non-Small Cell Lung Cancer: Results From a Phase 1 Study.
ESMO Congress 2024. Abstract 1257MO