Overall survival benefits of systemic therapy in the adjuvant setting are still unclear in clinical trials, and may need to be complemented with real-world studies
Based on data from clinical trials and real-world studies presented at the ESMO Congress 2024 (Barcelona, 13–17 September), questions remain on overall survival benefits in stage III melanoma.
Long-term data from clinical trials show that adjuvant therapy improves relapse-free and distant metastases-free survival in patients with completely resected stage III melanoma. However, some questions remain unanswered. Real-world studies report greater benefits with targeted therapy than immunotherapy in patients with BRAFV600 mutations, but adjuvant treatment was not associated with significant improvement in overall or melanoma specific survival.
According to updated results from the EORTC 1325-MG/KEYNOTE-054 trial, efficacy benefits are maintained at 7 years with adjuvant pembrolizumab compared with placebo in 1,019 randomised patients with high-risk stage III melanoma (Abstract 1095P). The data support the previously published findings at 5 years (N Engl J Med. 2022;1:EVIDoa2200214).
At 7 years of follow-up, pembrolizumab resulted in a clinically meaningful improvement in relapse-free survival (RFS; primary endpoint; 50% versus 36%; hazard ratio [HR] 0.63; 95% confidence interval [CI] 0.53–0.74), distant metastasis-free survival (DMFS; 54% versus 42%; HR 0.64; 95% CI 0.54–0.76) and progression/recurrence-free survival 2 (PRFS2; 61% versus 53%; HR 0.69; 95% CI 0.57–0.84) compared with placebo. Improvements with pembrolizumab were consistent regardless of BRAF mutation or PD-L1 status.
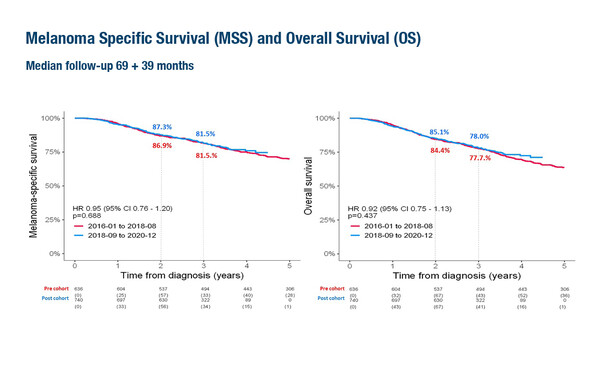
Overall survival (OS) data for adjuvant pembrolizumab are not yet available and are eagerly awaited. “Notably, there are currently no OS trial data available for adjuvant immunotherapy compared with placebo in this setting, and OS data from real-world studies suggest that the surrogate endpoints reported in adjuvant clinical trials may not translate into an OS benefit in clinical practice,” highlights Dr Teresa Amaral from the Skin Cancer Center, Tübingen, Germany. OS data are the focus of a Swedish registry-based study presented at the Congress involving 1,376 patients with stage III sentinel lymph node-positive cutaneous melanoma (Abstract 1079MO). Patients were divided into two cohorts depending on the date of diagnosis relative to the introduction of adjuvant therapy in Sweden in 2018: January 2016 to August 2018 (pre-cohort) or September 2018 to December 2020 (post-cohort). At a median follow-up of 3 years in the cohort diagnosed after the introduction of adjuvant therapy, there was no evidence of a melanoma specific or overall survival benefit.
“It is interesting to note that most patients who did not receive adjuvant therapy in the post-cohort were those with stage IIIA disease and/or sentinel lymph node metastasis <1 mm (64%). Indeed, these are patients for whom there is low to no evidence of benefit from adjuvant therapy. Also of interest is that most of the patients (81%) received adjuvant immunotherapy,” notes Amaral. “Probably not all patients treated in this real-world setting would have been eligible for clinical trials so we can only indirectly compare the data. Still, this is very much needed information that adds to the discussion about adjuvant therapy in stage III melanoma.” As the last follow-up of the patients from the Swedish registry was at the end of 2022, an update would provide further insight.
Additional real-world data were presented at the Congress in a 4-year follow-up of a multicentre cohort study (DeCOG) of 589 patients with stage III melanoma (Abstract 1124P). Two-year data were previously reported (Eur J Cancer. 2023;191:112957). Among patients with BRAF-mutated melanoma (n=232), the risk of recurrence was greater for patients receiving PD-1 inhibitors than for those treated with targeted therapy (HR 1.57; 95% CI 1.09–2.26). “Consistent with other real-world studies, these data suggest that patients with a BRAF mutation may derive greater RFS benefit from targeted therapy than immunotherapy. It is interesting to note that here 47% of patients with a BRAF mutation were treated with targeted therapy, allowing some level of comparison between targeted and immunotherapy in patients with BRAF mutation,” says Amaral. “As with the previous study, we also need to be mindful that these are real-world data and not a head-to-head comparison as in a randomised clinical trial.” While the risk of relapse was significantly higher with anti-PD-1 versus targeted treatment in BRAF-mutated disease, no clinically meaningful difference was reported in melanoma-specific survival. “Patients treated with immunotherapy had more relapses than those receiving targeted therapy, but could still benefit from subsequent therapies, resulting in a similar survival,” comments Amaral.
At this point, are the improvements in surrogate efficacy endpoints sufficient to warrant reimbursement of adjuvant treatments for melanoma? “It is important to say that some of the patients included in these studies would now be candidates for neoadjuvant immunotherapy with or without adjuvant therapy, which was not available at the time the studies were recruiting. On the other hand, for patients with microscopic disease, neoadjuvant therapy would not be a possibility, and for them adjuvant therapy is available.” Amaral considers that it is important for patients to have a longer RFS, as this is time when patients are free of disease, and do not need to receive therapy. Looking into the future what we need is to have biomarkers that can identify the patients who need and will most likely benefit from adjuvant therapy, beyond the American Joint Committee on Cancer classification.
Programme details
Eggermont AMM, et al. Pembrolizumab versus placebo after a complete resection of high-risk stage III melanoma: 7-year results of the EORTC 1325-MG/KEYNOTE-054 double-blind phase 3 trial. ESMO Congress 2024, Abstract 1095P
Poster Display – Melanoma and other skin tumours, 14.09.2024, h. 12:00 – 13:00, Hall 6
Livingstone E, et al. Adjuvant treatment of patients with stage III melanoma: 4-year follow-up time of multicenter real-world study. ESMO Congress 2024, Abstract 1124P
Poster Display – Melanoma and other skin tumours, 14.09.2024, h. 12:00 – 13:00, Hall 6
Helgadottir H, et al. No survival benefit after the introduction of adjuvant treatment in stage III melanoma: a nationwide registry-based study. ESMO Congress 2024, Abstract 1079MO
Mini Oral Session – Melanoma and other skin tumours, 15.09.2024, h. 14:45 – 16:15, Oviedo Auditorium – Hall 3