Neoadjuvant immunotherapy combinations were associated with high long-term survival rates and were superior to adjuvant nivolumab in patients with resectable melanoma, according to a phase III study and analysis of pooled studies
Neoadjuvant therapy is the standard of care for resectable stage ≥IIIB melanoma, and considerable benefits have recently been observed with immunotherapy in this setting. In updated findings from two key studies presented at the ESMO Congress 2024 (Barcelona, 13–17 September), long-term survival benefits were reported with various neoadjuvant immunotherapy combinations.
Unprecedented and lasting survival benefit was reported in a survival update (median follow-up 3 years) from the International Neoadjuvant Melanoma Consortium; the analysis consisted of 818 patients with stage ≥IIIB melanoma who received neoadjuvant therapy in a clinical trial (77%) or routine care (23%) setting (LBA41). Event-free survival (EFS) at 3 years was 81% with anti-PD-1 combined with anti-LAG3 therapy, 77% with anti-PD-1 combined with anti-CTLA-4 and 64% with anti-PD-1 therapy alone. The highest 3-year relapse-free survival (RFS) rate (89%) was observed in patients who achieved a major pathological response (MPR; 333/574 [58%] of those who received an immune checkpoint inhibitor [ICI]).
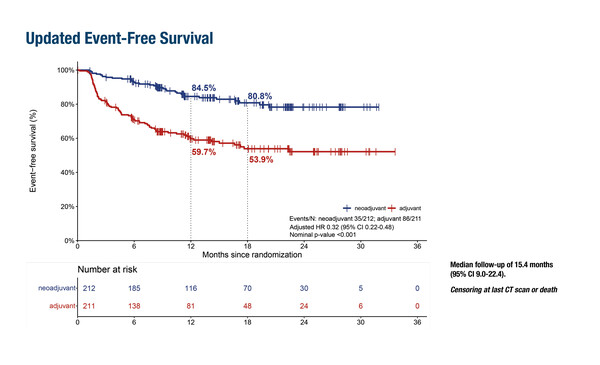
High rates of EFS and distant metastasis-free survival (DMFS) have also been reported with a neoadjuvant nivolumab plus ipilimumab combination in patients with macroscopic stage IIIB or IIIC melanoma (n=212) in an update from the phase III NADINA trial (LBA42). At 18 months of follow-up, EFS was 80.8% and DMFS was 85.7%. These rates were significantly higher than those observed in patients who received adjuvant nivolumab (n=211): EFS: 53.9% (hazard ratio [HR] 0.32; 95% confidence interval [CI] 0.22–0.48; p<0.001); DMFS: 62.4% (HR 0.37; 95% CI 0.24–0.57; p<0.001).
Patients who achieved a MPR, radiological complete response (CR) or partial response (PR) achieved the highest RFS and DMFS rates at 18 months; MPR: 93.1% and 96.0%; CR: 88.8% and 92.3%; PR: 93.0% and 95.6%, respectively.
Programme details
Long GV, et al. Long-term survival with neoadjuvant therapy in melanoma: updated pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). ESMO Congress 2024, LBA41
Proffered Paper Session – Melanoma and other skin tumours, 14.09.2024, h. 10:15 – 11:45, Salamanca Auditorium – Hall 5
Lucas MW, et al. Distant metastasis-free survival of neoadjuvant nivolumab plus ipilimumab versus adjuvant nivolumab in resectable, macroscopic stage III melanoma: the NADINA trial. ESMO Congress 2024, LBA42
Proffered Paper Session – Melanoma and other skin tumours, 14.09.2024, h. 10:15 – 11:45, Salamanca Auditorium – Hall 5