Novel targeted strategies are investigated in uveal melanoma, especially for those who do not respond to the bispecific fusion protein, with some encouraging early-phase data from c-MET/PKC inhibitors
Metastatic uveal melanoma (mUM) has historically been characterised by poor overall survival (OS) and a lack of effective treatment options. However, a breakthrough occurred in 2022 with the regulatory approval of the bispecific fusion protein tebentafusp as a treatment for patients with HLA-A*02:01-positive mUM. At the ESMO Congress 2023 (Madrid, 20–24 October), the latest findings on tebentafusp were presented, including 3-year survival data from the pivotal phase III trial and insights from a real-world study.
Tebentafusp was approved based on a survival benefit in patients with previously untreated HLA-A*02:01-positive mUM in the phase III IMCgp100-202 trial (N Engl J Med. 2021;385:1196–1206). Long-term follow-up data from this study were presented at the Congress, revealing a 3-year OS rate of 27% for tebentafusp compared with 18% for patients treated with the investigator’s choice of therapy (mostly single-agent pembrolizumab, some ipilimumab or dacarbazine) (LBA50). Commenting on these findings, Prof. Bastian Schilling from the University of Wuerzburg, Germany, says, “It is important to have additional data from registration trials to see if treatment benefits are sustained. The survival difference at the 3-year timepoint in this study is only 9%, suggesting that a small proportion of patients gain long-term benefit from tebentafusp. Identifying predictive biomarkers may enable us to see greater efficacy with this treatment by enriching the study population with patients who are most likely to benefit.”
The effectiveness of tebentafusp for mUM in a real-world setting was also evaluated, in which respective tumour reduction and disease control were reported for 5 (14%) and 23 (64%) patients of 36 who received treatment at a single centre in Canada (Abstract 1131P). The 1-year OS and progression-free survival (PFS) rates were 68% and 14%, respectively, and PFS and time to treatment discontinuation (TTD) were, respectively, 6 and 9 months. More than half of patients (53%) were able to reduce the frequency of their outpatient visits from once weekly to once every 3 weeks after a median of 6 doses. In addition, premedications were successfully stopped for 53% of patients after a median of 7 doses. “The efficacy data in this study are similar to those reported in the pivotal phase III trial,” comments Schilling. “It is also interesting that they report TTD as a clinical endpoint, as many patients continue to receive treatment despite having disease progression.” He also notes, “Weekly tebentafusp infusions can be inconvenient for patients and it is interesting that the investigators in this study have attempted to optimise the treatment regimen. However, we need to be cautious when changing the established schedule, as the impact on treatment outcomes is unknown.”
There is clearly a need to identify other druggable targets and alternative treatment options for the wider mUM population, including those with HLA-A*02:01-negative disease not eligible for tebentafusp. The majority of patients with mUM harbour driver mutations in the genes for heterotrimeric G proteins, GNAQ/GNA11, leading to protein kinase C (PKC) activation. In addition, the c-MET tyrosine kinase receptor has previously been implicated in the pathogenesis of UM. Therapies targeting these proteins are therefore of interest for the treatment of mUM and some clinical activity for the oral selective PKC inhibitor, darovasertib, was reported at the Congress (Abstract 1081O), which confirm previously reported findings (Br J Cancer. 2023;128:1040–1051).
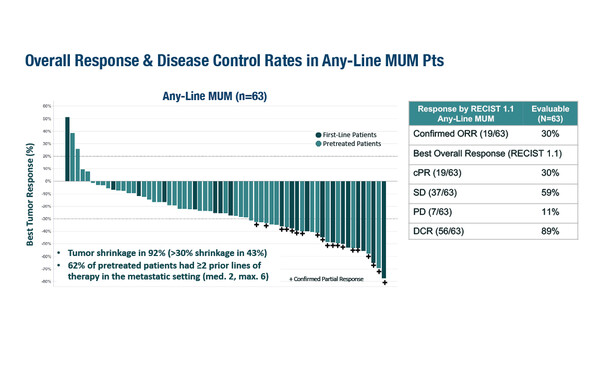
In a phase I/II study of darovasertib plus the c-MET inhibitor crizotinib, 30% of 63 evaluable patients had a confirmed partial response, with 92% displaying tumour shrinkage. The median PFS was 6.8 months and clinical efficacy was observed in both HLA-A*02:01-positive and -negative patients. In addition, there were deep and sustained circulating tumour DNA (ctDNA) responses and the safety profile was manageable. “First-generation PKC inhibitors were poorly tolerated and it is pleasing to see that tolerability is much better for the second-generation inhibitors such as darovasertib,” says Schilling. “The clinical activity in this study is also very promising. These results strongly advocate for the further development of this combination for mUM.”
Abstracts discussed:
Piperno-Neumann S, et al. Three-year survival with tebentafusp in previously untreated metastatic uveal melanoma in a phase 3 trial. ESMO Congress 2023, Abstract LBA50
Mini Oral Session – Melanoma and other skin tumours, 21.10.2023, h. 14.45 – 16.10, León Auditorium – Hall 7
Ribeiro MF, et al. Management of metastatic uveal melanoma (MUM) patients on tebentafusp in a real-world setting. ESMO Congress 2023, Abstract 1131P
Poster Display Session – Melanoma and other skin tumours, 22.10.2023, h. 12.00 – 13.00, Hall 8
Mckean M, et al. ctDNA reduction and clinical efficacy of the darovasertib + crizotinib (daro + crizo) combination in metastatic uveal melanoma (MUM). ESMO Congress 2023, Abstract 1081O
Proffered Paper Session – Melanoma and other skin tumours, 23.10.2023, h. 08.30 – 10.00, Valencia Auditorium – Hall 10