Updated data from two studies report significant improvements in recurrence-free survival and distant-metastasis free survival with pembrolizumab or nivolumab
Adjuvant therapy using PD-1 blockade with pembrolizumab or nivolumab is the current standard of care in the management of stage IIB or higher melanoma, with the two agents approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) based on the results of KEYNOTE-716 and CheckMate 76K studies (Lancet. 2022 Apr 30;399(10336):1718-1729; Nat Med. 2023 Nov;29(11):2835-2843). As presented at the ESMO Congress 2024 in September, updated data from these two studies confirm the key role of anti-programmed cell death protein 1 (PD-1)-based immunotherapy in the adjuvant setting for patients with melanoma.
In the double-blind, randomised, placebo-controlled phase III KEYNOTE-716 study, adjuvant pembrolizumab showed to significantly prolong recurrence-free survival (RFS) and distant-metastasis free survival (DMFS) compared to placebo in patients with resected stage IIB or IIC melanoma A total of 976 patients were randomised to either receive intravenous pembrolizumab (200 mg) or placebo once every three weeks for ≤17 cycles in Part 1. Participants who completed the initial treatment cycles and experienced disease recurrence were eligible for rechallenge with or to switch over to pembrolizumab at the same dose and schedule for up to 35 cycles (up to ~2 years) in Part 2.
After more than four years of follow-up, updated data reported a prolonged RFS (HR, 0.62; 95% CI, 0.50-0.78) and DMFS (HR, 0.59; 95% CI, 0.45-0.77) in the pembrolizumab arm compared to placebo (71% vs 58% and 81% vs 70%, respectively), and a promising progression/recurrence-free survival 2 (PRFS2) was observed (Abstract 1078MO). In Part 2, 9 patients in the pembrolizumab group were rechallenged, and 71 patients in the placebo group crossed over to pembrolizumab, and no new safety signals were observed with a pembrolizumab rechallenge or crossover. At data cutoff, overall survival (OS) data were immature.
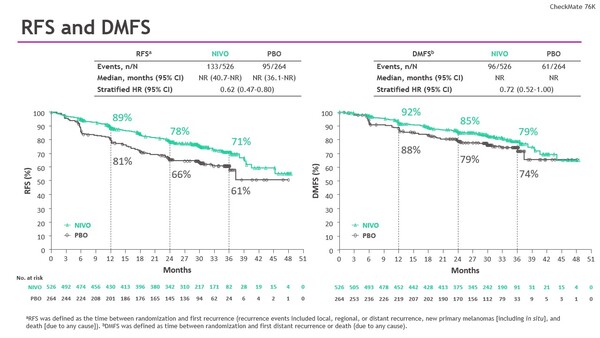
The phase III, double-blind CheckMate 76K trial involved 790 patients with resected stage IIB/C melanoma to either receive nivolumab 480 mg or placebo every four weeks for 12 months, and an improvement in RFS and DMFS with nivolumab was previously reported. 3-year results presented at the Congress (median follow-up of 34.2 months) showed a reduced risk of recurrence or death (HR, 0.62; 95% CI, 0.47–0.80) and a reduced risk of distant metastasis (HR, 0.72; 95% CI, 0.52–1.00) with nivolumab compared to placebo regardless of pre-specified patient subgroup (Abstract 1077MO). A benefit of nivolumab was observed in preliminary PFS2 data (HR, 0.71; 95% CI, 0.48–1.06), suggesting that its use in the adjuvant setting does not negatively impact subsequent therapy. No new safety signals were observed.
Abstracts discussed at ESMO Congress 2024
Long GV et al. Adjuvant nivolumab v placebo in stage IIB/C melanoma: 3-year results from CheckMate 76K.
ESMO Congress 2024, Abstract 1077MO
Luke JJ et al. Pembrolizumab (pembro) vs placebo as adjuvant therapy for high-risk stage II melanoma: Long-term follow-up, rechallenge, and crossover in KEYNOTE-716.
ESMO Congress 2024, Abstract 1078MO