New study findings support this treatment strategy as a new standard of care for patients with high-risk, locally advanced cervical cancer
Encouraging data revealed in a Presidential presentation at the ESMO Congress 2024 (Barcelona, 13–17 September) indicate a significant overall survival (OS) benefit for patients with newly diagnosed locally advanced cervical cancer receiving pembrolizumab and concurrent chemoradiotherapy (CRT) (Abstract 709O).
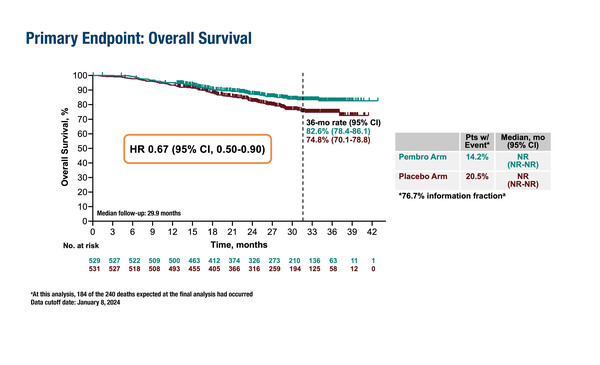
In a second interim analysis from the ENGOT-cx11/GOG-3047/KEYNOTE-A18 randomised, double-blind, phase III study involving 1,060 patients, the 36-month OS rate was 82.6% for pembrolizumab plus concurrent CRT and 74.8% for placebo plus concurrent CRT (hazard ratio [HR] 0.67; 95% confidence interval [CI], 0.50–0.90; p=0.0040). Median OS was not reached in either treatment arm. The survival benefit was observed at a median follow-up of 29.9 months, and was consistent across all pre-specified subgroups, including International Federation of Gynecology and Obstetrics (FIGO) 2014 stages IB2–IIB (HR 0.89; 95% CI 0.55–1.44) and III–IVA (HR 0.57, 95% CI, 0.39–0.83). Grade ≥3 treatment-related adverse events were reported in 69.1% of patients in the pembrolizumab plus concurrent CRT arm and in 61.3% of patients in the placebo plus concurrent CRT arm.
Prof. Remi Nout from the Erasmus MC Cancer Institute, Rotterdam, Netherlands, comments on the significance of these findings, stating, “These results are highly relevant for this high-risk population, offering a greater chance of cure compared with existing treatments. The high-risk patient population included in this study came from a diverse geographical and healthcare background and this inclusivity adds to the robustness and applicability of the findings.” However, Nout has reservations about the duration of treatment. “It should be noted that the treatment has to be administered over a 2-year period, which increases both costs and the burden on patients, but overall, the benefits likely outweigh the risks,” he adds.
Previously, the first interim analysis from the ENGOT-cx11 study had revealed a significant improvement in progression-free survival for patients with persistent, recurrent or metastatic cervical cancer receiving pembrolizumab plus concurrent CRT (Lancet. 2024;403:P1341–1350), which led to the regulatory approval of this combination for patients with FIGO 2014 stage III–IVA cervical cancer.
Regarding the global application of pembrolizumab plus concurrent CRT, Nout says, “At present the cost of treatment is a major concern and realistically, it is unlikely to be affordable for patients or healthcare systems in lower- and middle-income countries, where cervical cancer is most prevalent.” He also emphasises the necessity for modern radiotherapy techniques. “Using a modern approach likely contributed to the successful delivery of this treatment and its manageable side-effect profile. Older techniques may not yield the same benefits and could lead to an increase in side effects. In general, promoting the availability and use of modern techniques is crucial for increasing the feasibility and long-term success of this treatment,” he concludes.
Programme details
Lorusso D, et al. Pembrolizumab plus chemoradiotherapy for high-risk locally advanced cervical cancer: Overall survival results from the randomized, double-blind, phase III ENGOT-cx11/GOG-3047/KEYNOTE-A18 study. ESMO Congress 2024, Abstract 709O
Presidential Symposium I – Practice-changing trials , 14.09.2024, h. 16:30 – 18:15, Barcelona Auditorium – Hall 2