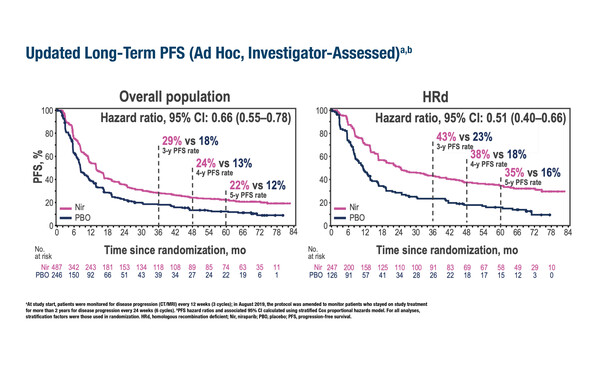
Patients with homologous-recombination-deficient advanced ovarian cancer alive at 5 years were twice as likely to be progression free with niraparib than placebo, although no long-term overall survival benefit was reported
At the ESMO Congress 2024 (Barcelona, 13–17 September), final overall survival (OS) data after a median follow-up of approximately 6 years revealed no benefit of niraparib maintenance over placebo for patients newly diagnosed with advanced ovarian cancer at high risk of recurrence in the phase III PRIMA/ENGOT-OV26/GOG-3012 study (LBA29). The median OS was 46.6 months with niraparib and 48.8 months with placebo (hazard ratio [HR] 1.01) in the overall population, and 71.9 months with niraparib and 69.8 months with placebo (HR 0.95) in patients with homologous-recombination-deficient (HRd) tumours.
Previously, PRIMA had met its primary endpoint, demonstrating significantly prolonged progression-free survival (PFS) for first-line niraparib maintenance versus placebo in patients with advanced ovarian cancer who responded to first-line platinum-based chemotherapy, regardless of whether they had HRd tumours (N Engl J Med. 2019;381:2391–2402).
Ad-hoc analysis results presented at the Congress show that 5-year PFS in the overall population was 22% for niraparib and 12% for placebo. For patients with HRd tumours alive at 5 years, there was a striking difference in PFS results, with patients in the niraparib arm twice as likely to be progression free than patients in the placebo arm (35% versus 16%, respectively). In the HRd patient population, the median time to first subsequent therapy was prolonged in the niraparib arm compared with the placebo arm (26.9 months versus 13.9 months, respectively; HR 0.55). Niraparib also prolonged the median time to first subsequent therapy in the overall population compared with placebo (17.0 months versus 12.0 months, respectively; HR 0.74). No new safety signals were reported.
Programme details
González-Martín A, et al. Final overall survival (OS) in patients with newly diagnosed advanced ovarian cancer (OC) treated with niraparib first-line (1L) maintenance: results from PRIMA/ENGOT-OV26/GOG-3012. ESMO Congress 2024, LBA29
Proffered Paper Session – Gynaecological cancers, 14.09.2024, h. 14:45 – 16:15, Sevilla Auditorium – Hall 2