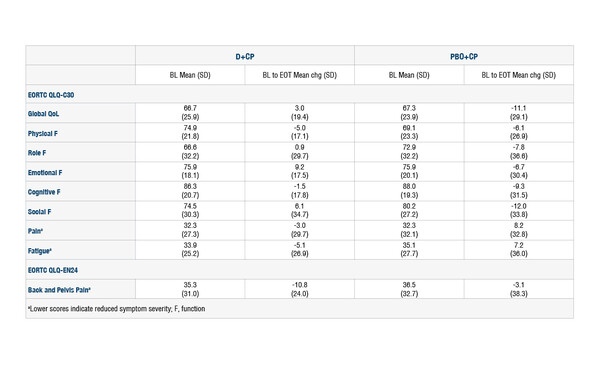
Data from selected patients in the RUBY trial show improvements in QoL domains over the course of treatment and benefits compared with placebo
According to patient-reported outcomes (PROs) results presented at the ESMO Congress 2023 (Madrid, 20–24 October), dostarlimab plus chemotherapy was associated with improvements over placebo plus chemotherapy in the 118 patients in the RUBY trial with mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) primary advanced or recurrent endometrial cancer (Abstract 749P). Over the course of treatment, improvements were reported in global quality of life (QoL) (3.0), emotional function (9.2), social function (6.1) and pain (−3.0) – as assessed using mean changes from baseline to the end of treatment in EORTC QLQ-C30 scores. An improvement in back/pelvis pain was also seen, as shown by a mean reduction of −10.8 in the EORTC QLQ-EN24 score from baseline to the end of treatment. In patients receiving placebo plus chemotherapy, back/pelvis pain was the only one of the domains tested with either QoL tool to show an improvement between baseline and the end of treatment (−3.1).
Comparison of the treatment arms demonstrated significant improvements with dostarlimab versus placebo in the change from baseline to end of treatment for QoL (least-squares means 14.7 [standard error 5.45]; p=0.008), role function (12.7 [5.92]; p=0.032), emotional function (14.3 [4.92]; p=0.004), social function (13.5 [5.43]; p=0.014) and fatigue (−13.3 [5.84]; p=0.025).
The RUBY trial randomised patients 1:1 to treatment with dostarlimab plus carboplatin/paclitaxel or placebo plus carboplatin/paclitaxel every 3 weeks for 6 cycles followed by dostarlimab or placebo monotherapy every 6 weeks for up to 3 years or until disease progression. PRO assessments were administered at baseline, day 1 of each cycle and at all follow-up visits.
The PRO findings support phase III data published earlier this year at the ESMO Virtual Plenary in March, which reported that dostarlimab plus carboplatin/paclitaxel significantly improved progression-free survival compared with placebo plus chemotherapy in patients with primary advanced or recurrent endometrial cancer, with particular benefits in the subgroup of patients with dMMR/MSI-H tumours (N Engl J Med. 2023;388:2145–2158).
Abstract discussed:
Valabrega G, et al. Patient-reported outcomes (PROs) in patients (pts) with mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) primary advanced or recurrent endometrial cancer (pA/rEC) in the ENGOT-EN6-NSGO/GOG3031/RUBY trial. ESMO Congress 2023, Abstract 749P
Poster Display Session – Gynaecological cancers, 22.10.2023, h. 12.00 – 13.00, Hall 8