Results from four early-phase studies show that combining immune checkpoint inhibitors with other immunotherapies or with TKIs is a potentially efficacious strategy for the treatment of different diseases including GIST and vascular sarcomas
New treatment strategies are urgently required to address the poor prognosis of advanced sarcoma. Although responses to immune checkpoint inhibitors (ICIs) are often limited in these tumours, there is growing interest in the benefits of combining ICIs with other targeted therapies. Several presentations at the ESMO Congress 2023 (Madrid, 20–24 October) report preliminary data supporting these treatment combinations in patients with sarcoma.
In a Mini Oral Session, the outcomes from a phase Ib study of the Fc-enhanced anti-CTLA-4 antibody botensilimab combined with the PD-1 inhibitor balstilimab in 50 patients with refractory metastatic sarcoma were presented (Abstract 1919MO). After a median follow-up of 5.7 months, the objective response rate (ORR) among 41 efficacy-evaluable patients was 17% (95% confidence interval [CI] 7–32) according to RECIST v1.1 and 20% (95% CI 9–35) according to iRECIST, and the corresponding median durations of response were 11.8 months and 19.4 months. The ORR was highest with higher botensilimab doses: 29% with 2 mg/kg and 11% with 1 mg/kg (according to RECIST v1.1). The disease control rate at 6 months was 24% and the 6-month progression-free survival (PFS) rate was 37% according to RECIST v1.1. The safety profile was consistent with previously reported data for these agents. “This study is encouraging as the ORR in the highest botensilimab dose group is greater than that previously reported for the combination of an anti-CTLA-4 antibody and a PD-1 inhibitor, perhaps reflecting the enhanced Fc function of botensilimab,” says Dr Tom Wei-Wu Chen from the National Taiwan University Hospital, Taipei, Taiwan. “Also, it included histologies such as leiomyosarcoma and visceral angiosarcomas, which rarely respond to ICIs.”
In a second study, the phase II REGOMUNE trial, promising efficacy is suggested for the combination of regorafenib, a multi-kinase inhibitor, plus the PD-L1 inhibitor avelumab in advanced or metastatic gastrointestinal stromal tumours (GIST) (Abstract 1920MO). Of 46 patients who received treatment and were assessable for efficacy, 4 (6.5%) had a partial response (PR), 31 (67.4%) had stable disease (SD) and 10 (21.7%) had progressive disease (PD). Median PFS and overall survival (OS) were 5.5 months and 17.7 months, respectively, and 29.3% of patients were progression-free at 1 year. “The median PFS is slightly longer than was observed with regorafenib monotherapy in the phase III GRID study in a similar patient population (Lancet. 2013;381:295–302). However, the proportion of patients who are progression-free at 1 year in the REGOMUNE trial is particularly interesting,” highlights Chen. “There appears to be a subset of this population who are achieving a long-term benefit from this combination. What is needed now is to identify biomarkers that will allow us to determine which patients with GIST are most likely to respond to an ICI.”
Favourable data were also presented for the combination of the TKI sunitinib plus the PD-1 inhibitor nivolumab in 26 patients with vascular sarcomas enrolled in the phase II IMMUNOSARC II study (Abstract 1922P). Among 15 patients with angiosarcomas who were evaluable for efficacy assessments, the median PFS was 3.9 months and the 6-month PFS rate was 36%. Five patients (33%) had a PR, 7 patients (47%) had SD and 3 (20%) had PD. Median OS was 15.7 months. Commenting on these findings, Chen says, “There is definitely a role for combining ICIs with anti-angiogenic TKIs in angiosarcoma. The data reported from this study are complementary to the highly encouraging results previously reported from the Alliance A091902 study of cabozantinib plus nivolumab in patients with advanced angiosarcoma (J Clin Oncol. 2023;41(16_suppl):11503).” Considering the heterogeneity of angiosarcomas, he adds, “It will be interesting to see whether there is a differential response to this combination for angiosarcomas from different primary sites, e.g., skin, head and neck, or visceral angiosarcomas.”
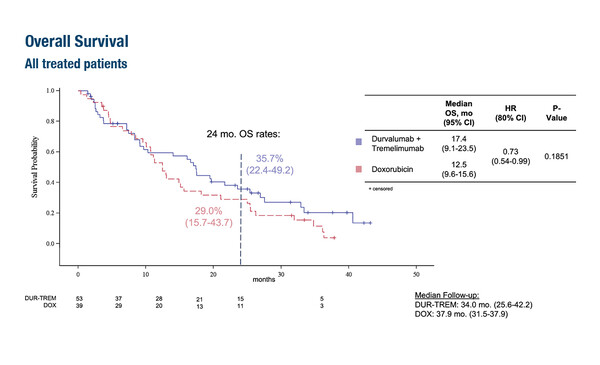
Finally, results from the MEDISARC phase II trial revealed similar clinical activity for the combination of the PD-L1 inhibitor durvalumab plus anti-CTLA-4 antibody tremelimumab compared with doxorubicin in 103 treatment-naïve patients with advanced or metastatic histologically-confirmed soft tissue sarcomas (LBA90). The respective median PFS and 6-month PFS with durvalumab plus tremelimumab versus doxorubicin were 2.7 months versus 2.8 months (hazard ratio [HR] 1.22; 95% CI 0.90–1.64; p=0.405) and 11.4% versus 33.6%. Median OS – the study’s primary endpoint – was 17.4 months with durvalumab plus tremelimumab and 12.5 months with doxorubicin (HR 0.73; 95% CI 0.54–0.99; p=0.185). The 2-year OS rate was 35.7% with durvalumab plus tremelimumab and 29.0% with doxorubicin.
The most frequent soft tissue sarcomas in the respective durvalumab plus tremelimumab and doxorubicin treatment arms were leiomyosarcoma (34% and 18%), unclassified sarcoma (25% and 13%) and adipocytic sarcoma (11% and 26%).
Adverse events (AEs) occurred at a similar frequency with durvalumab plus tremelimumab (90.6%) and doxorubicin (89.7%), but AEs of grade ≥3 were more common with the combination (52.8% versus 41.0%, respectively). Commenting on the study, Chen says, “While the ORR and PFS data for durvalumab plus tremelimumab were a little disappointing, the trend for better OS in the durvalumab plus tremelimumab versus doxorubicin arm suggest there may be a carry-over effect of the ICI combination or specific histology or subgroups that would benefit from the first-line ICI treatment.”
Chen concludes that these four studies support a role for ICIs in the treatment of sarcoma, either combined with other immunotherapies or with TKIs. Looking to the future, Chen notes that, “Randomised controlled trials will be needed to confirm the efficacy shown in these studies. It will also be useful to identify predictive biomarkers that may allow us to determine which subsets of patients are likely to gain the most benefit from these combinations of agents. Also, a lack of knowledge remains regarding the optimal dose of anti-angiogenic agents when used in combination with ICIs. We need to identify the dose that allows us to maximise tolerability without compromising efficacy.”
Abstracts discussed:
Wilky B, et al. Efficacy and safety of botensilimab (BOT) plus balstilimab (BAL) in patients (pts) with refractory metastatic sarcoma. ESMO Congress 2023, Abstract 1919MO
Mini Oral Session – Sarcoma, 21.10.2023, h. 10.15 – 11.45, Toledo Auditorium – Hall 3
Cousin S, et al. Regomune: A phase II study of regorafenib + avelumab in solid tumors – Results of the advanced or metastatic gastrointestinal stromal tumors (mGIST) cohort. ESMO Congress 2023, Abstract 1920MO
Mini Oral Session – Sarcoma, 21.10.2023, h. 10.15 – 11.45, Toledo Auditorium – Hall 3
Gruenwald V, et al. A randomized phase II study of durvalumab and tremelimumab compared to doxorubicin in patients with advanced or metastatic soft tissue sarcoma (MEDISARC, AIO-STS-0415). ESMO Congress 2023, LBA90
Proffered Paper Session – Sarcoma, 22.10.2023, h. 08.30 – 10.00, Valencia Auditorium – Hall 10
Hindi Muñiz N, et al. Immunosarc II master trial: phase II of sunitinib and nivolumab in vascular sarcomas cohort – A GEIS, ISG and UCL study. ESMO Congress 2023, Abstract 1922P
Poster Display Session – Sarcoma, 23.10.2023, h. 12.00 – 13.00, Hall 8