Study findings indicate that the agent is promising as maintenance therapy, but managing its tolerability presents challenges
Results presented at the ESMO Congress 2024 (Barcelona, 13–17 September) demonstrated that the multikinase inhibitor regorafenib delayed disease progression in patients with metastatic or locally advanced soft tissue sarcomas.
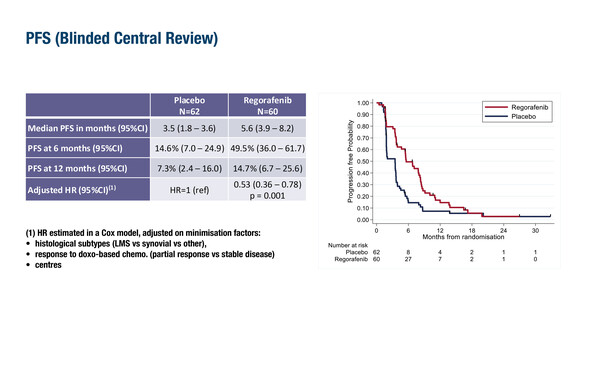
For patients with soft tissue sarcomas, there is still an unmet need for an effective maintenance treatment to sustain the response to first-line doxorubicin and to delay the initiation of second-line therapy. The EREMISS phase II trial randomised 127 patients who had previously achieved a partial response or stable disease after first-line doxorubicin-based chemotherapy to either regorafenib (n=65) or placebo (n=62) (Abstract 1718O). Median progression-free survival (PFS; defined as time from randomisation to first progression), the primary endpoint, was significantly longer in the regorafenib arm compared with the placebo arm (5.6 months versus 3.5 months; hazard ratio [HR] 0.53; 95% confidence interval [CI] 0.36–0.78); p=0.001). However, no significant difference in median overall survival was observed between patients treated with regorafenib compared with those receiving placebo (27.6 months versus 20.5 months; HR 0.78; 95% CI 0.50–1.22; p=0.28). Grade ≥3 adverse events occurred in 60.9% of patients in the regorafenib arm compared with 11.3% of those in the placebo arm. Overall, 18 (28.1%) patients discontinued regorafenib due to toxicity.
“These results are promising, with the study meeting its primary endpoint of a significant improvement in PFS,” says Dr Claudia Valverde of Vall d'Hebron University Hospital, Barcelona, Spain. However, she has some doubts about the tolerability of the regimen. “The high percentage of patients discontinuing regorafenib is concerning and highlights the toxicity issues associated with this agent, even at reduced doses (120 mg/day), as in the study. Although the data are not directly comparable, there is some evidence that the chemotherapy agent trabectedin may be less toxic as maintenance therapy than regorafenib in this setting (Lancet Oncol. 2022;23:1044–1054). Before regorafenib can be implemented into clinical practice, additional data will be required, particularly regarding its impact on time to disease progression after second-line therapy, to avoid exposing patients to unnecessary toxicity,” cautions Valverde.
Looking ahead, there are additional data that are sought in this setting. “Since patients may need to receive maintenance therapies for an extended period of time, future studies must include data on quality of life. In addition, the variability in treatment response underscores the need to identify the patient subgroups most likely to benefit from a particular treatment,” she concludes.
Programme details
Penel N, et al. EREMISS trial: A double-blind placebo (PBO)-controlled randomised trial assessing efficacy/safety of regorafenib (REGO) as maintenance therapy after 1st line doxorubicin-based chemotherapy in advanced soft-tissue sarcoma (ASTS) patients (pts). ESMO Congress 2024, Abstract 1718O
Proffered Paper Session – Sarcoma, 16.09.2024, h. 14:45 – 16:15, Santander Auditorium –Hall 5