Scarcity of prospective data was reported as well as a limited use of real-world evidence as definitive evidence to regulatory approval decisions of targeted therapies in Europe
Despite a growing interest in real-world data (RWD) to generate real-world evidence (RWE) for guiding treatment decisions in oncology, results presented at the ESMO Congress 2023 (Madrid, 20–24 October) highlight that the quality of RWD from clinical trials is still a big limitation to their reliability and use in regulatory drug approval processes.
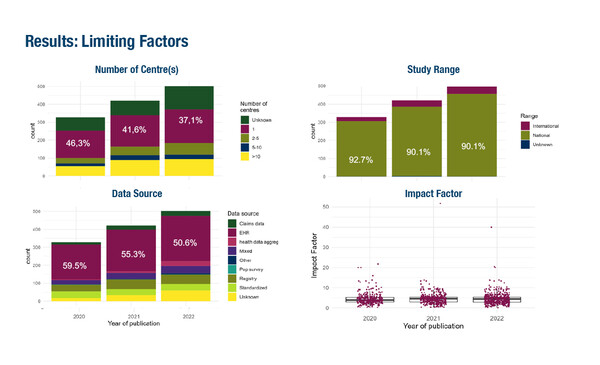
By systematically reviewing RWE PubMed publications on oncology targeted therapies between 2020 and 2022, experts from the ESMO Real World Data and Digital Health Working Group highlighted an increasing number of RWE studies – from 328 in 2020 to 502 in 2022 – but noted that their impact is still limited (Abstract 1689O). Among 1,251 eligible, non-interventional studies identified for solid tumours treated with targeted therapies, only 8% were international studies, the majority were single-centre studies (40%) and only 3% were published in high-impact journals.
“In this study, most RWE data published as recently as 3 years ago was still retrospective and based on medical records,” says Prof. Massimo Di Maio from the University of Turin, Italy. “We know that retrospective data can be unreliable, particularly in terms of safety – when lower-grade, but potentially clinically impactful side-effects may not be captured – and this can produce a misleading representation of a treatment’s effects.”
According to Di Maio, to avoid the inevitable biases of retrospective data, future studies should plan for the prospective collection of information. It is expected that with advances in technology and the widespread availability of electronic health records, prospective data collection on a collaborative basis between centers will become a new routine. “For targeted therapies, which in clinical practice are generally used in only a few patients in any given centre, this should provide the opportunity to obtain reliable, real-world, real-time data from a clinically meaningful number of patients,” continues Di Maio. “However, for easy and quick extraction of data from patients’ electronic health records to the study database, compatibility of data collection software – which currently varies widely between centres – will be paramount.”
Specific guidance on the design, submission, and assessment of RWE (Canada’s Drug and Health Technology Agency; European Network for Health Technology Assessment) has been developed by research corporations and regulatory and health technology assessment (HTA) agencies. However, they do not cover all the various peculiarities in oncology research, including the increasing use of technological innovations like artificial intelligence, thus making it even more difficult for researchers to fully unveil the potential of RWD.
Quality and reliability issues of RWE also result in its current use as supporting rather than definitive evidence in the regulatory approval of targeted anticancer therapies, as reported in an analysis screening in Europe (Abstract 1702P). According to the findings, among 55 oncology targeted therapies with regulatory decisions made by the EMA between 2018–2022, RWE was a consideration in deliberations in 22/75 (29%) European Public Assessment Reports (EPARs), increasing from 23% in 2018–2020 to 39% in 2021–2022. In 19/22 EPARs (86%), RWE was used in the pre-authorisation setting; 20 RWE studies were supportive of the regulatory decision in 15 of these EPARs, eight RWE studies were non-supportive in seven EPARs but none were definitive in the regulatory approval of a targeted agent. Of 14 RWE studies published, most originated from data aggregators (n=5), followed by patient registries or health records (n=3 each), standardised cohort data (n=2) or combined sources (n=1).
The study results confirm findings from another analysis indicating that RWE data are generally of insufficient quality to inform clinical practice (Eur J Cancer. 2021;155:136–144). However, according to Di Maio the reported marked increase in the contribution of RWE to approvals over the past 5 years is a positive signal although not surprising. “There is a call for RWE both from clinicians, who want reassurance that the results from clinical trials are replicated in the clinical setting, and from regulatory agencies, which are actively encouraging the use of RWE. The data presented illustrate that we are progressing towards a more systematic approach to RWE use for regulatory purposes,” he says.
The recognition that RWE can provide important supplementary and complementary information to that obtained in randomised clinical trials, including in underrepresented patient populations, is particularly true for targeted therapies. “Clinical trials for targeted therapies differ from the traditional phase III designs we are used to seeing for regulatory approval,” he concludes. “Given the limited number of patients and single-arm nature of these trials, RWE – not just in the pre-authorisation setting but also post-approval – can help to answer questions not addressed in the trials and so provide a comprehensive view of the efficacy and safety of a new agent.”
Abstracts discussed:
Pellat A, et al. Comprehensive mapping review of real-world evidence publications focusing on targeted therapies in solid tumors: a collaborative work from ESMO Real World Data and Digital Health Working Group. ESMO Congress 2023, Abstract 1689O
Proffered Paper Session: Policy and preventive strategies, 21.10.2023, h. 14:45 – 16:15, Cádiz Auditorium – NCC
Derksen JWG, et al. Real-world evidence contributions to European Medicines Agency’s safety and efficacy evaluations of oncology targeted therapies between 2018-2022. ESMO Congress 2023, Abstract 1702P
Poster Display Session – Policy and preventive strategies, 22.10.2023, h. 12.00 – 13.00, Hall 8