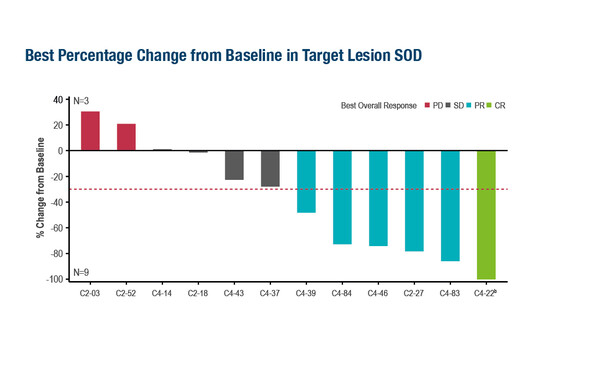
Antitumour responses were consistent with those previously reported for a broader population of patients with advanced melanoma
Results from the phase II C-144-01 study presented at the ESMO Congress 2023 (Madrid, 20–24 October) showed clinically meaningful and durable antitumour activity of lifileucel, an autologous, centrally manufactured tumour-infiltrating lymphocyte agent, in patients with advanced mucosal melanoma who had progressed on or after anti-PD-1/PD-L1 therapy (Abstract 1086MO). After a median follow-up of 35.7 months, the confirmed objective response rate (ORR) was 50.0% (95% confidence interval [CI] 21.1–78.9) in a study subgroup of 12 patients with this difficult-to-treat cancer. Median duration of response (DoR) was not reached, with 4 of 6 responders having durable and ongoing responses at data cutoff.
A median 26.1 × 109 (range 3.3–72) tumour-infiltrating lymphocytes (TILs) were infused, followed by a median of 5.5 (range 3–6) interleukin-2 (IL-2) doses. Safety was consistent with the known safety profiles of non-myeloablative lymphodepletion and IL-2. Febrile neutropenia (58.3%) and hypotension (33.3%) were the most common grade 3–4 non-haematological treatment-emergent adverse events. Grade 3–4 haematological laboratory abnormalities reflected non-myeloablative lymphodepletion, with neutropenia, leukopenia, lymphocytopenia and thrombocytopenia reported for all 12 patients; 66.7% experienced anaemia.
Patients in this mucosal melanoma subgroup had a lower tumour mutational burden (TMB) than patients with cutaneous melanoma (mean TMB 2.145 mut/Mb [n=4] versus 10.47 mut/Mb [n=47], respectively), although 12-month TIL persistence was similar for patients with mucosal or cutaneous melanoma. The antitumour responses observed were consistent with those reported previously for the overall population with advanced melanoma in the C-144-01 study (J Immunother Cancer. 2022;10:e005755). Taken together, these findings further support the potential benefit of one-time lifileucel treatment; however, as discussed in an editorial by Dr Marco Donia, TIL therapy is currently only available at a small number of specialised centres worldwide, generally through clinical trials or early access programmes, thus limiting its access to patients who may benefit from the treatment.
Abstract discussed:
Grigoleit G-U, et al. Lifileucel tumor-infiltrating lymphocyte (TIL) cell therapy in patients (pts) with advanced mucosal melanoma after progression on immune checkpoint inhibitors (ICI): Results from the phase 2 C-144-01 study. ESMO Congress 2023, Abstract 1086MO
Mini Oral Session – Melanoma and other skin tumours, 21.10.2023, h. 14:45 – 16:10, León Auditorium – Hall 7