The PARP inhibitor shows durable clinical benefits in HRD+ patients with advanced disease reinforcing the importance of testing for BRCA mutations and/or HRD status.
Positive long-term data from two phase III trials of olaparib maintenance treatment in patients with newly diagnosed advanced ovarian cancer were reported at ESMO Congress 2022.
The phase III SOLO1/GOG-3004 trial evaluated maintenance treatment with olaparib versus placebo in patients with BRCA-mutated advanced ovarian cancer, with the latest analysis reporting overall survival (OS) data after 7 years of follow-up (Abstract 517O). At an OS data maturity of 38.1% in March 2022, median OS was not reached with olaparib compared with 75.2 months with placebo (hazard ratio [HR] 0.55; 95% confidence interval [CI] 0.40–0.76).
“These OS data are highly clinically meaningful,” says Prof. Jonathan Ledermann, from UCL Cancer Institute, London, UK. “Positive progression-free survival (PFS) data were previously reported after 5 years of follow-up (Lancet Oncol. 2021;22:e539), and now at the 7-year point we can see a clear difference in OS. With only a handful of patients starting a new line of treatment between 5 and 7 years, there is increasing evidence that patients may benefit from this treatment, but longer follow-up is needed to confirm this.”
Historically, the prognosis for patients with advanced ovarian cancer has been very poor and over the last decade it has become clear that PARP inhibitors are active not only in patients with BRCA mutations but also in those with defects in homologous recombination repair of DNA. Early work demonstrated significant benefit in recurrent ovarian cancer, changing the treatment landscape and more recent studies have been focussed on first-line treatment, which included patients without a BRCA mutation. “Improvements in PFS with PARP inhibitor maintenance treatment have been established, but it is unclear if these benefits will translate into an improved OS with longer follow-up,” highlights Ledermann.
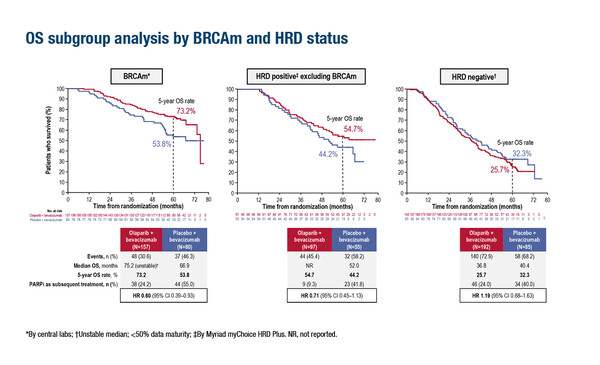
Final OS data were presented from a second study, the phase III PAOLA-1/ENGOT-ov25 trial of olaparib plus bevacizumab – an antiangiogenic agent – versus bevacizumab alone as maintenance treatment in patients who responded to platinum-based chemotherapy plus bevacizumab (LBA29). In the overall population, median OS was 56.5 months with olaparib plus bevacizumab and 51.6 months with bevacizumab alone (HR 0.92; 95% CI 0.76–1.12; p=0.4118). In an exploratory analysis, median OS improved with olaparib plus bevacizumab over bevacizumab alone in homologous recombination deficiency positive (HRD+) patients, regardless of BRCA mutation status (HRD+ and BRCA mutation: HR 0.60; 95% CI 0.39–0.93; HRD+ excluding BRCA mutation: HR 0.71; 95% CI 0.45–1.13). No benefit in OS was seen in patients who were negative for HRD.
Discussing these data, Ledermann says, “Bevacizumab plus chemotherapy followed by bevacizumab maintenance is associated with delayed time to progression and in many countries, it is a standard of care. If a patient has a BRCA mutation, by definition they will be HRD+, but not all HRD+ patients will harbour BRCA mutations. The positive OS data in HRD+ patients – even when patients with BRCA mutations are excluded – are particularly interesting and are effectively broadening the group of patients who may benefit from addition of olaparib.” Ledermann adds, “There is an emerging change in practice towards increased testing for HRD and these results are likely to reinforce this.”
In both trials presented, the safety data are encouraging. “One of the concerns with PARP inhibitors is the small risk of developing myelodysplastic syndrome (MDS) or acute myeloid leukaemia (AML), particularly in patients with a BRCA mutation,” comments Ledermann. “The low levels of MDS and AML in both trials with longer follow-up is reassuring.”
To conclude, Ledermann says, “Results from the SOLO1/GOG-3004 and PAOLA-1/ENGOT-ov25 trials reinforce the importance of testing for BRCA mutations and/or HRD status and demonstrate that HRD+ patients can experience long-term benefits with PARP inhibitors. The 7-year follow-up in patients with a BRCA mutation raises the possibility that olaparib could increase treatment rates. However, there is a great need for research into treatment options for patients with HRD-negative disease.”
Abstract Presented:
Ray-Coquard I, et al. Final overall survival (OS) results from the Phase III PAOLA-1/ENGOT-ov25 trial evaluating maintenance olaparib (ola) plus bevacizumab (bev) in patients (pts) with newly diagnosed advanced ovarian cancer (AOC).ESMO Congress 2022, LBA 29
Proffered Paper Session – Gynaecological cancers, 09.09.2022, h. 14:00 – 15:30, Fécamp Auditorium