Addition of the PD-L1 inhibitor atezolizumab to chemotherapy plus bevacizumab showed little or no benefit in the relapsed setting, echoing previous data from front-line therapy
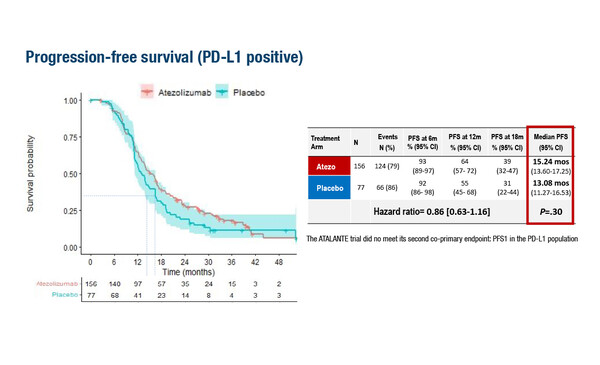
The ATALANTE/ov29 trial met neither of its two co-primary endpoints – delivering a non-significant trend towards improvement in median progression-free survival (PFS) of approximately 2 months in both the intention-to-treat (ITT) population and the PD-L1-positive subset (LBA30). Results presented at ESMO Congress 2022 were disappointing but perhaps not surprising, as Prof. Nicoletta Colombo, Director of the Ovarian Cancer Centre at the European Institute of Oncology, Milan, Italy, comments. “We have seen similar results in the front-line setting, in the IMagyn050 trial (J Clin Oncol. 2021;39:1842–1855), where there was also no PFS benefit in either the ITT population or PD-L1-positive subgroup when atezolizumab was added to the standard of care,” she says.
The ATALANTE/ov29 trial randomised patients to atezolizumab (1200 mg on day 1 q3w or 800 mg on days 1 and 15 q4w) with carboplatin and pegylated liposomal doxorubicin (CbD; n=410) or placebo and chemotherapy of physician’s choice (CbD, carboplatin and gemcitabine or carboplatin and paclitaxel; n=204), plus bevacizumab (15 mg/kg on day 1 q3w or 10 mg/kg on days 1 and 15 q4w). Investigator-assessed median PFS in the ITT population was 13.54 months versus 11.27 months in the atezolizumab and placebo arms, respectively; corresponding data for PD-L1-positive patients were 15.24 months versus 13.08 months. In neither group was PFS significantly improved by addition of azetolizumab.
In both the ATALANTE/ov29 and IMagyn050 trials, the cut-off to denote PD-L1 positivity was ≥1%. Colombo theorises: “The ≥1% threshold for denoting PD-L1 positivity may be too low. In the IMagyn050 trial a pre-specified exploratory subgroup analysis using a higher threshold of ≥5% – which is the threshold used in urothelial carcinoma – produced an interesting signal of efficacy.”
So where do we go from here? Is the strategy of adding anti-angiogenic therapy and immune checkpoint inhibition to backbone chemotherapy a flawed one? Colombo thinks it is too early to say. “While it was not a primary endpoint, preliminary data on overall survival from the ATALANTE/ov29 study look promising and it will be interesting to see how those develop when data are mature,” she notes. And when asked if a combination of immune checkpoint inhibition and PARP inhibition is likely to be more effective, Colombo thinks it could be. “There is a strong rationale for combining an immune checkpoint inhibitor (ICI) with a PARP inhibitor since PARP inhibitors result in up-regulation of PD-L1,” she says. “Up to now, studies of PARP inhibitors with ICI have not yielded striking results, at least not in the platinum-resistant setting, and so adding an anti-angiogenic may make a difference. This is because PARP inhibition increases circulating angiogenic factors and that is why it may be wise to use all three agents together – a PARP inhibitor, an ICI and an anti-angiogenic,” Colombo concludes.
Abstract discussed:
Kurtz JE, et al. Phase III ATALANTE/ov29 trial: Atezolizumab (Atz) versus placebo with platinum-based chemotherapy (Cx) plus bevacizumab (bev) in patients (pts) with platinum-sensitive relapse (PSR) of epithelial ovarian cancer (OC). ESMO Congress 2022, LBA30
Proffered Paper Session: Gynaecological cancers, 09.09.2022, h. 14:00 – 15:30, Fécamp Auditorium