Results from two studies shows promise for combination immunotherapy in a population with a high unmet need for new treatments
At ESMO Congress 2022, new data presented confirm the promise of immunotherapy in metastatic or recurrent cervical cancer, with clinical efficacy reported regardless of PD-L1 expression.
In a first study, the CheckMate 358 trial, results indicate that durable tumour regression with manageable toxicity can be achieved with a combination of nivolumab and ipilimumab in patients with advanced disease (Abstract 520MO).
The phase I/II CheckMate 358 trial was designed to evaluate the efficacy and safety of nivolumab and ipilimumab combination therapy in patients with recurrent or metastatic cervical cancer. Patients in this study received either nivolumab monotherapy (n=19) or the combination of nivolumab plus ipilimumab at two different schedules and dosages (nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks [N3I1, n=45] or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for 4 cycles followed by nivolumab 240 mg every 2 weeks [N1I3, n=112]). After a median follow-up of 30.4 months, patients treated with N1I3 had a higher investigator-assessed objective response rate – the primary endpoint – compared to those treated with N3I1 or nivolumab monotherapy (38.4% vs 31.1% vs 26.3%, respectively). The median duration of response was 24.4 months and 34.1 months for patients treated with N3I1 or N1I3, respectively, and was not reached for patients treated with nivolumab monotherapy. Median overall survival was 21.6 months (95% confidence interval [CI] 8.3–46.9), 15.2 months (95% CI 9.0–36.2) and 20.9 months (95% CI 14.4–32.8), and median progression-free survival was 5.1 months (95% CI 1.9–9.1), 3.8 months (95% CI 2.1–10.3) and 5.8 months (95% CI 3.8–9.3) for patients treated with nivolumab monotherapy, N3I1 or N1I3, respectively.
“An important point is that these response rates were achieved regardless of PD-L1 expression,” comments Dr Domenica Lorusso from the Fondazione Policlinico Gemelli IRCCS, Catholic University of Rome, Italy, putting the results in context. “This is vitally important because pembrolizumab has been approved only for tumours that are positive for PD-L1, whereas for this combination we now have evidence that the responses are not linked to PD-L1 expression.” She also discusses how the results can be affected by the extent to which a patient is pre-treated. “The responses were higher in patients who were less heavily pre-treated, but patients who had received two or more prior lines of treatment still had a response rate of 34.9%. This is an amazing result compared to what would have been achieved for these patients with chemotherapy.” Regarding toxicity, despite it being increased with combination therapy, it seemed manageable.
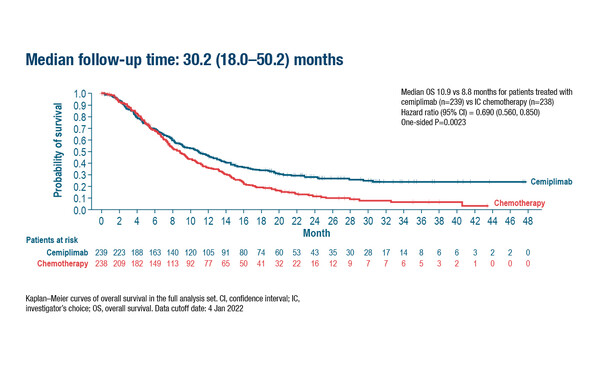
At the same Mini Oral session at ESMO Congress 2022, final OS results from a phase III trial evaluating the long-term efficacy of immunotherapy in patients with recurrent or metastatic cervical cancer were also presented (Abstract 519MO). The EMPOWER-Cervical 1/GOG-3016/ENGOT-CX-9 trial evaluated cemiplimab 350 mg IV every 3 weeks (n=304) versus single-agent chemotherapy (investigator’s choice; n=304) for up to 96 weeks. After a median follow-up of 30.2 months, OS – the primary endpoint – in patients stratified by squamous cell carcinoma histology was 10.9 months with cemiplimab and 8.8 months with chemotherapy (hazard ratio [HR] 0.69; 95% CI 0.56–0.85; p=0.0023), and in the overall population was 11.7 months and 8.5 months, respectively (HR 0.66; 95% CI 0.55–0.79; p<0.00001).
“There is currently a huge unmet clinical need for novel therapies or treatment strategies for advanced cervical cancer,” says Lorusso highlighting that for patients who have relapsed following first-line platinum-based chemotherapy, outcomes are very poor regardless of which second-line drug is used. “Immunotherapy is an attractive treatment strategy for cervical cancer as these tumours are strongly linked to human papillomavirus infection and are frequently associated with high expression of PD-L1 and T-cell infiltration,” she adds. However, although there is growing evidence to support immunotherapy in cervical cancer, the response rate is still less than 15% when it is used as monotherapy (Gynecologic Oncology. 2021;162:S27).
Looking to the future, Lorusso anticipates an increasing use of immunotherapy in patients with cervical cancer, potentially in combination with chemoradiation or chemotherapy as a first-line treatment. "Also, antibody-drug conjugates will become a very important class of agents for cervical cancer, with tisotumab vedotin-tftv having already received regulatory approval in the United States based on the results from the innovaTV 204 clinical trial (Lancet Oncol. 2021;22:609-619),” she concludes.
Abstracts discussed:
Oaknin A, et al. Safety and efficacy of nivolumab (NIVO) ± ipilimumab (IPI) in patients (pts) with recurrent/metastatic cervical cancer (R/M CX CA) in CHECKMATE 358. ESMO Congress 2022, Abstract 520MO
Mini Oral Session 10.09.2022, h. 08:30 – 10:00, Évry Auditorium
Oaknin A, et al. Phase 3 EMPOWER-Cervical 1/GOG-3016/ENGOT-CX9 trial of cemiplimab in recurrent or metastatic (R/M) cervical cancer: long-term survival analysis. ESMO Congress 2022, Abstract 519MO
Mini Oral Session 10.09.2022, h. 08:30 – 10:00, Évry Auditorium