Latest findings on the use of single-agent and combination immunotherapy are not conclusive, despite being promising
Encouraging results from four studies discussed at the ESMO Congress 2021 still do not provide a final answer to how, when and where immune checkpoint inhibitors (ICIs) should be used in the management of neuroendocrine neoplasms (NENs). In particular, it is not yet completely certain whether ICI as combination therapy can clearly improve outcomes in NENs over ICI as monotherapy, irrespective of grade and primary site. “These considerations are in line with the conflicting results and the low-level of evidence reported in the studies with ICI,” says Dr Francesca Spada from the European Institute of Oncology, Milan, Italy. “In the rare tumour setting, a study design that incorporates centralised pathological review and clinically and biologically homogenous patient populations in terms of primary sites and biological features, for example, would strengthen the level of evidence produced and could enable primary endpoints to be met.”
One critical issue is the need for a robust study design to gain a greater understanding of the true value of immunotherapy in rare tumours, such as NENs.
Presented today, updated pathological review-confirmed results from the phase II DUNE (GETNE 1601) trial revealed that combination durvalumab plus tremelimumab was associated with a 9-month overall survival (OS) rate of 36.1% and median OS of 5.9 months (95% confidence interval [CI] 2.0–9.7) in NENs (Abstract 1107P). Around one-third (30%) of the total 33 patients with grade 3 disease had prolonged survival for over 12 months after initiation of therapy with durvalumab plus tremelimumab, regardless of tumour differentiation, Ki67 level or PD-L1 expression. These encouraging results in patients with high-grade neoplasms follow on from previous observations of only moderate activity for the combination therapy in the heavily pre-treated all-comer population of 123 patients with NENs of gastroenteropancreatic (GEP) or lung origin who had progressed on standard therapies (Ann Oncol 2020;31(Suppl 4):S711-S724).
“These new, pathologically-confirmed data for the grade 3 cohort contribute to better-understand the population in the study, which were composed of 54.5% with neuroendocrine carcinomas (NECs) and 45.5% with grade 3 neuroendocrine tumours (NETs), and to reinforce the improved outcomes of 9.1% ORR by immune-related RECIST assessment and prolonged OS of over 1 year in one-third of these patients,” says Spada, highlighting that patient selection is key to the efficacy of the combination treatment. “By selecting the patient population carefully and enhancing the study design, the authors have been able to show that the combination of durvalumab plus tremelimumab in grade 3 NENs produces more clear and convincing response data than we saw in previously published results.”
A second presentation of results from DUNE suggested a role for sequential therapies, including immunotherapy, in advanced NENs (Abstract 1099MO). In the investigation of the activity of systemic therapies after progression on immune checkpoint inhibitors, objective response rate (ORR) and progression-free survival (PFS) on later systemic therapies were longer than on initial durvalumab plus tremelimumab in high-grade NENs.
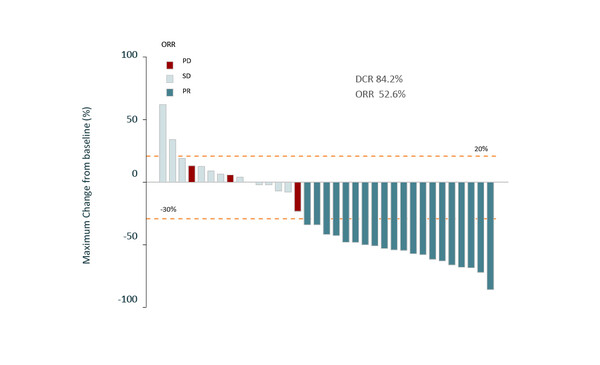
Preliminary results from the phase II NICE-NEC trial (GETNE T1913) indicate that adding nivolumab to platinum-doublet chemotherapy may be a promising strategy in the first-line therapy of grade 3 NENs (Abstract 1098O). At a median follow-up of 8.2 months, disease control rate was 84% and ORR was 53% in 38 patients with unresectable, locally advanced or metastatic grade 3 NENs of the GEP tract or unknown origin. Median PFS and 6-month PFS rate were 5.7 months (95% CI 5.1–7.9) and 39% (95% CI 26–60), respectively. Final survival results are awaited. Commenting on the trial design, Spada says that the results of this study are interesting. However, she is disappointed that an additional study arm was not included in NICE-NEC with chemotherapy alone: “If a checkpoint inhibitor with chemotherapy is being assessed, we also need to be able to evaluate the activity and efficacy both of the nivolumab plus platinum-doublet chemotherapy combination and the platinum-doublet chemotherapy alone,” she says.
Also presented today were results from the non-comparative phase II GCO-001 NIPINEC study, showing that the addition of ipilimumab to nivolumab doubled the primary endpoint of ORR at 8 weeks compared with nivolumab alone (14.9% [95% CI 8.2–24.2] and 7.2% [95% CI 2.7–15.1], respectively) in 185 pre-treated patients with advanced, pulmonary or GEP poorly differentiated NENs refractory to chemotherapy (LBA41). However, survival results were similar with the single agent and the combination: median PFS and OS were 1.8 months (95% CI 1.7–2.0) and 7.2 months (95% CI 3.7–14.1), respectively with nivolumab and 1.9 months (95% CI 1.6–2.1) and 5.8 months (95% CI 3.4–7.6), respectively with nivolumab plus ipilimumab.
Discussing all these results in the context of quality of life for patients, Spada notes, “The main grade 3–4 side-effects of this combination were manageable. Now more than ever, oncologists are accustomed to managing the toxicity of ICI, so tolerability should not be problematic for most patients, but we need to get the study design right to understand the real role and place of immunotherapy in NENs.”
Capdevila, J et al. Durvalumab plus tremelimumab in patients with grade 3 neuroendocrine neoplasms of gastroenteropancreatic origin: updated results from the multicenter II DUNE trial (GETNE 1601). ESMO Congress 2021, Abstract 1107P
Hernando J et al. Durvalumab plus tremelimumab influence on response to subsequent treatments in patients with neuroendocrine neoplasms (NENs) of gastroenteropancreatic and lung origins: Results from the phase II DUNE trial (GETNE 1601). ESMO Congress 2021, Abstract 1099MO
Mini oral session – NETs and endocrine tumours, 20.09.2021, h. 17:55 – 18:00, Channel 5
Walter T et al. Nivolumab (nivo) ± ipilimumab (ipi) in pre-treated patients with advanced, refractory pulmonary or gastroenteropancreatic poorly differentiated neuroendocrine tumors (NECs) (GCO-001 NIPINEC). ESMO Congress 2021, Abstract LBA41
Proffered Paper session – NETs and endocrine tumours, 21.09.2021, h. 13:30 – 13:40, Channel 3
Riesco-Martinez MC et al. Nivolumab plus platinum-doublet chemotherapy as first-line therapy in unresectable, locally advanced or metastatic G3 neuroendocrine neoplasms (NENs) of the gastroenteropancreatic (GEP) tract or unknown (UK) origin: Preliminary results from the phase II NICE-NEC trial (GETNE T1913). ESMO Congress 2021, abstract 1098O
Proffered Paper session – NETs and endocrine tumours, 21.09.2021, h. 13:40 – 13:50, Channel 3