Practice-changing results were also presented in bronchopulmonary and non-pancreatic NETs with somatostatin analogues and VEGFR inhibitors
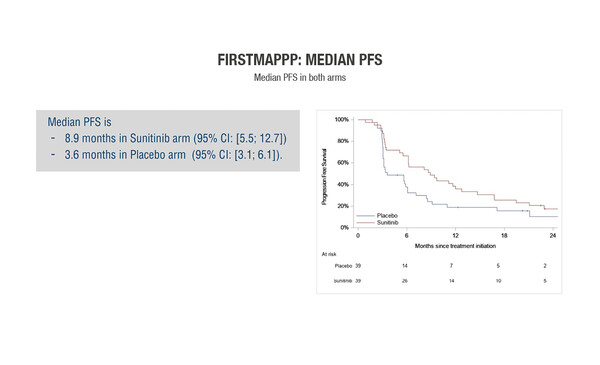
Results from the FIRSTMAPPP study presented at yesterday’s Presidential Symposium 3 provide the highest level of evidence ever achieved for the benefit of sunitinib in patients with malignant progressive phaeochromocytoma and paraganglioma (MPP) (Abstract 567O_PR). This is the first academic, randomised, double-blind trial investigating the tyrosine kinase inhibitor (TKI) sunitinib in patients with these very rare cancers. In patients receiving sunitinib, the 12-month progression-free survival (PFS) rate – the primary endpoint of the study – was 35.9%, meeting the prespecified target increase from 20% to 40%. The 12-month PFS rate with placebo was 18.9%. Of the 78 patients recruited over a period of 8 years, 60% had received prior therapy.
“This is a strong result for a treatment based on sound mechanistic principles,” says Prof. Juan Valle, University of Manchester and Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester, UK. “None of the treatment options we currently have for advanced MPP are supported by randomised clinical trial evidence. Although the placebo arm in FIRSTMAPPP was included as an internal control, to ensure the validity of statistical assumptions, we can see that the 12-month PFS rate with sunitinib was nearly double that seen with placebo.” Perhaps as important is the good tolerability of sunitinib. “MPP is commonly treated with cyclophosphamide/vincristine/dacarbazine, all quite old agents and all very toxic. Sunitinib will be much better tolerated.”
According to findings from the phase III randomised SPINET trial, treatment with somatostatin analogues (SSAs) may be extended beyond gastrointestinal (GI) NETs to the treatment of bronchopulmonary (BP) NETs (Abstract 1096O). Among 77 patients receiving lanreotide autogel/depot for advanced somatostatin receptor-positive BP NETs, the median PFS was 16.6 months and 13.6 months with placebo. The PFS benefit of lanreotide over placebo was greater among patients with typical carcinoid (TC) tumours (median PFS 21.9 months versus 13.9 months, respectively) than in those with atypical carcinoid (AC) tumours (median PFS 13.8 months versus 11.0 months, respectively). Enrolment to the study was stopped due to slow accrual. According to Valle, “The authors’ conclusions – that lanreotide may be particularly useful for patients with TC BP NETs – confirm what we know from GI NETS, where SSAs are used primarily for patients with well-differentiated low-grade tumours. So, the SPINET trial provides important consistency in the message that SSAs are an appropriate treatment option for more indolent NETs.”
These three trials provide evidence that the effects of the different types of treatment – TKIs, SSAs and VEGFR inhibitors – appear to be comparable across the spectrum of NETs subgroups.
Updated results from the phase II/III AXINET trial – comparing octreotide plus the selective vascular endothelial growth factor receptor (VEGFR) inhibitor axitinib with octreotide plus placebo in advanced progressive grade 1–2 extra-pancreatic NETs – using blinded independent central review (showing a median PFS of 16.6 months versus 9.9 months; hazard ratio 0.71; p=0.02) highlighted that there was a PFS advantage from axitinib when applying this robust methodology (Abstract 1097O). These results follow on from the data on investigator-assessed PFS presented at ASCO GI 2021, which reported no significant advantage for axitinib over placebo.
“In terms of clinical practice,” suggests Valle, “these three trials provide evidence that the effects of the different types of treatment – TKIs, SSAs and VEGFR inhibitors – appear to be comparable across the spectrum of NETs subgroups. The extension of SSA treatment to patients with lung NETs is in keeping with current practice in small intestinal NETs and pancreatic NETs. And the AXINET trial shows that VEGFR inhibition, which is currently limited to patients with pancreatic NETs, can be used in non-pancreatic NETs, including lung NETs.”
He concludes, “These presentations also give us valuable information about trial frameworks for patients with rare NETs. They confirm the ability of trials to both ask and answer efficacy questions in such patient populations and they highlight the crucial role of widespread collaboration in academic studies. We know that to access these treatments, patients need to be managed through expert centres. We need to facilitate this if we are to ensure that patients are given the opportunity to get these treatments and to be enrolled in the next generation of clinical trials for new therapies.”
Baudin E et al. FIrst International Randomized STudy in MAlignant Progressive Pheochromocytoma and Paraganliomas (FIRSTMAPPP): an academic double-blind trial investigating Sunitinib. ESMO Congress 2021, Abstract 567O_PR
Presidential symposium 3, 20.9.2021, h. 15:05 – 15:20, Channel 1
Horsch D et al. Lanreotide autogel/depot (LAN) in patients with advanced bronchopulmonary (BP) neuroendocrine tumors (NETs): results from the phase 3 SPINET study. ESMO Congress 2021, Abstract 1096O
Proffered Paper session – NETs endocrine tumours 21.9.2021, h. 14:10 – 14:20, Channel 3
Garcia-Carbonero R et al. The AXINET trial (GETNE1107): Axitinib plus octreotide LAR improves PFS by blinded central radiological assessment vs placebo plus octreotide LAR in G1-2 extrapancreatic NETs. ESMO Congress 2021, Abstract 1097O
Proffered Paper session – NETs endocrine tumours 21.9.2021, h. 14:20 – 14:30, Channel 3