The oncolytic herpes virotherapy does not show survival benefit in combination with pembrolizumab, but encouraging early data are reported with intratumoural injection of myeloid dendritic cells
The phase III MASTERKEY-265 study of the herpes simplex virus type-1-derived immunotherapy, talimogene laherparepvec (T-VEC), in combination with pembrolizumab in patients with advanced melanoma failed to meet its progression-free survival (PFS) primary endpoint, as presented today at the ESMO Congress 2021 (Abstract 1037O), despite earlier promising phase Ib results with this combination in this setting.
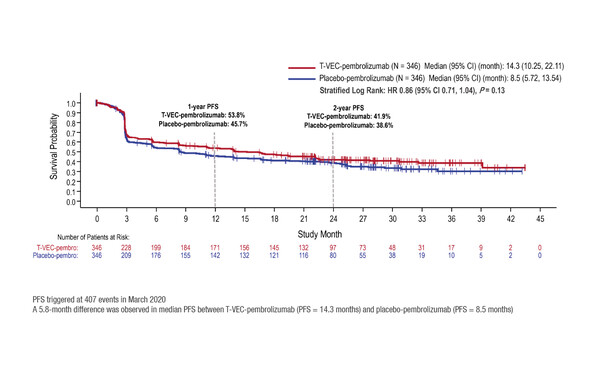
The MASTERKEY-265 study included 692 patients with unresectable, PD-1-naïve, advanced (stage IIIB–IVM1C) melanoma. At a median follow-up of 31.0 months, the hazard ratio (HR) for PFS – a dual primary endpoint along with overall survival (OS) – was 0.86 (95% confidence interval [CI] 0.71–1.04; p=0.13), with median PFS of 14.3 months for T-VEC plus pembrolizumab and 8.5 months with placebo plus pembrolizumab (Figure). Although OS data are not mature, a survival benefit was not reported at the interim analysis (median not yet reached with T-VEC plus pembrolizumab compared with 49.2 months with placebo plus pembrolizumab; HR 0.96, 95% CI 0.76–1.22; p=0.74). Significant OS improvements are not expected in the primary OS analysis.
It is possible that a future analysis or study focusing on patient subgroups with specific biomarkers may prove more informative in identifying patients who are more likely to benefit from the combination.
According to Dr Marco Donia of the Herlev and Gentofte Hospital, University of Copenhagen, Denmark, it is disappointing that the encouraging results from the early clinical studies have not translated into survival benefits in this larger phase III study. “However, this is sadly not the first time that this has happened in advanced melanoma. A similar story was seen with epacadostat plus pembrolizumab in the ECHO-301/KEYNOTE-252 study (Lancet Oncol. 2019 Aug;20(8):1083-1097), with no survival benefits shown versus placebo plus pembrolizumab,” he comments. “We can only speculate on the myriad potential reasons for the failure of these studies to reach their primary endpoints in the overall population, but it is possible that a future analysis or study focusing on patient subgroups with specific biomarkers may prove more informative in identifying patients who are more likely to benefit from the combination.”
Despite negative results in combination with pembrolizumab, T-VEC continues to have a role in the treatment of patients with melanoma, and novel investigational approaches to immunotherapy with T-VEC have demonstrated encouraging early results. For example, a phase I clinical trial of intratumoural injection of autologous CD1c (BDCA-1)+/CD141 (BDCA-3)+ myeloid dendritic cells (myDC) in combination with T-VEC in patients with advanced pre-treated melanoma was presented yesterday (Abstract 962MO).
Although only 13 patients were enrolled in the study, some antitumour activity was reported – 2 patients achieved a pathological complete remission, 1 patient had a partial response, and 2 patients had a mixed response – with regression occurring at both injected and non-injected sites. Commenting on the results, Donia says that caution is warranted. “In these patients with previous failure on immune checkpoint inhibitors – a population with a large unmet need – these early signs of systemic effects are promising, but while we can be optimistic, other studies are needed to confirm these findings before clinical practice can change.”
Explaining further, he concludes, “In the absence of comparative studies, a matter of debate remains if the intratumoural agents show similar efficacy. Many potential agents are in development, and at the moment the front-runner seems to be oncolytic virus therapies, such as T-VEC, but any clinical differences between these treatments need to be determined.”
Ribas A et al. MASTERKEY-265: A phase 3, randomized, placebo (PBO)-controlled study of talimogene laherparepvec (T) plus pembrolizumab (P) for unresectable stage IIIB-IVM1C melanoma (MEL). ESMO Congress 2021, Abstract 1037O
Proffered Paper session – Melanoma and other skin tumours, 18.09.2021, h. 14:10 – 14:20, Channel 4
Schwarze JK et al. A phase I clinical trial on intratumoral injection of autologous CD1C (BDCA-1)+/CD141 (BDCA-3)+ myeloid dendritic cells (myDC) in combination with talimogene laherparepvec (T-VEC) in patients with advanced pretreated melanoma. ESMO Congress 2021, Abstract 962MO
Mini oral session – Investigational immunotherapy, 17.09.2021, h. 17:40 – 17:45, Channel 2