Immunotherapy was associated with a significant reduction in the risk of disease recurrence compared with placebo in patients with melanoma
The first interim analysis of KEYNOTE-716, presented today at Presidential Symposium I at the ESMO Congress 2021, suggests that adjuvant pembrolizumab is superior to standard-of-care observation for adult and children with more than 12 years with completely resected high-risk melanoma.
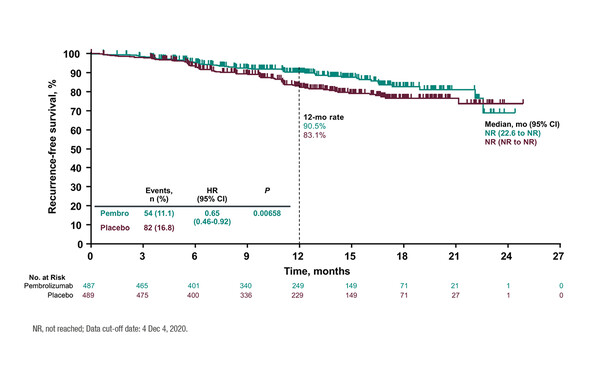
With a primary endpoint of investigator-assessed recurrence-free survival (RFS), the phase III, double-blind trial reported that adjuvant pembrolizumab significantly reduced the risk of disease recurrence by 35% compared with placebo (hazard ratio 0.65; 95% confidence interval 0.46–0.92; p=0.00658) at a median follow-up of 14.4 months (LBA3; Figure). Median RFS times were not reached in either arm. Pembrolizumab led to a reduction in the recurrence rate compared with placebo (11.1% versus 16.8%) and an almost halving of the number of distant recurrence events (23 versus 38). The 12-month RFS rate was 90.5% with immunotherapy and 83.1% with placebo.
The 976 patients aged ≥12 years in the trial were randomised equally to pembrolizumab 200 mg (2 mg/kg for paediatric patients) or placebo every 3 weeks for 17 cycles, up to 1 year. Randomisation was stratified in adults by T category (3b, 4a, 4b) and by a separate stratum for paediatric patients. Most patients had stage IIB disease (64%), with 34.8% having stage IIC disease.
The incidence of grade ≥3 adverse events (AEs) was higher with pembrolizumab than with placebo, both for any-cause AEs (25.9% versus 17.1%) and for drug-related AEs (16.1% versus 4.3%). Altogether, 15.3% of patients discontinued pembrolizumab due to a drug-related AE, compared with 2.5% receiving placebo. There were no deaths due to any-cause or drug-related AEs in the pembrolizumab arm, but four patients receiving placebo died due to any-cause AEs. Immune-mediated AEs (irAEs) were reported in 36.2% of patients receiving pembrolizumab and 8.4% of patients receiving placebo. The most common irAEs were hypothyroidism (15.7% with pembrolizumab versus 3.5% with placebo) and hyperthyroidism (10.4% versus 0.6%, respectively); most were grade 1–2 in severity.
Patients with resected stage IIB and IIC melanoma are at substantial risk of recurrence and KEYNOTE-716 is the first phase III, randomised, double-blind study to show that the anti-PD-1 pembrolizumab in the adjuvant setting significantly reduces the risk of recurrence compared with placebo in high-risk resected stage II disease.
Luke JJ et al. Pembrolizumab versus placebo after complete resection of high-risk stage II melanoma: Efficacy and safety results from the KEYNOTE-716 double-blind phase III trial. ESMO Congress 2021, Abstract LBA3
Presidential symposium 1, 18.9.2021, h. 16:05 – 16:20, Channel 1