Since its approval, tebentafusp is changing the treatment landscape for patients with metastatic uveal melanoma, but many questions remain
Uveal melanoma is the most common intraocular cancer in adults, representing approximately 3–5% of all melanomas (Nat Rev Dis Primers. 2020;6:24). Up to 50% of patients with primary uveal melanoma will develop metastases. Tebentafusp, a bispecific gp100 peptide-HLA-directed CD3 T-cell engager, emerged as the first systemic treatment option showing overall survival (OS) benefit in metastatic uveal melanoma (mUM), and was approved by the US FDA and EMA in early 2022 based on favourable results compared with investigator’s choice of therapy from the randomised phase III IMCgp100-202 trial in previously untreated mUM (202 Study) (N Engl J Med. 2021;385:1196–1206). Research is ongoing to establish how to integrate tebentafusp into the checkpoint-based clinical management of patients with this rare malignancy.
At ESMO Congress 2022, a single-centre retrospective cohort study explored the optimum treatment sequence of tebentafusp and immune checkpoint inhibitors (ICIs; anti-PD-1 +/- anti-CTLA-4) in patients with mUM (Abstract 831P). Among 10 patients who received tebentafusp before ICIs (T-I) there was one partial response (PR), 3 patients had stable disease (SD) and 6 had progressive disease (PD). In 10 patients receiving ICIs before tebentafusp (I-T), there were 2 SD and 8 PD (p=0.63 between groups). Median progression-free survival from ICI initiation was significantly longer in the T-I cohort than in the I-T cohort (2.86 months; 95% confidence interval [CI] 2.37–not available [NA] versus 2.35 months; 95% CI 0.92–NA; p=0.046). There was no significant difference between the T-I and I-T cohorts in median OS from the start of the first treatment (21.47 months; 95% CI 15.78–NA versus 16.88 months; 8.52–NA; p=0.38). –NA; p=0.38).
“Treatment with tebentafusp before ICI might be the more favourable sequence and, from a biological perspective, it could be hypothesised that attracting T-cells towards the tumour with tebentafusp, followed by anti-PD-1 or the anti-PD-1/anti-CTLA-4 combination to activate T-cells, would be the logical order,” says Dr Kalijn Bol from Radboud University Medical Center, Nijmegen, Netherlands, acknowledging that it is difficult to conduct large clinical studies with such a rare tumour type. “Further comparative analyses based on a larger cohort will be useful to guide decisions on treatment sequences,” she adds.
Tebentafusp can only be used for a subset of patients who are HLA-A*02:01-positive – an estimated 40–50% of Caucasian patients (Cancers 2019;11,971). Patients must also be reasonably fit to receive this intensive treatment. Tebentafusp is not yet available in some countries, but can be given in the Netherlands via an expanded access programme.
“Checkpoint inhibitors have never shown OS benefit in a phase III clinical trial in uveal melanoma. Since they are approved for cutaneous melanoma, they are given in several countries as standard treatment for mUM, but there is officially no standard treatment for those patients,” notes Bol. As checkpoint inhibitors have shown response rates for some patients with mUM, although low compared to cutaneous melanoma, Bol believes they should be considered for HLA-A*02:01-negative patients with mUM as there remains an unmet need for specific treatment for these patients.
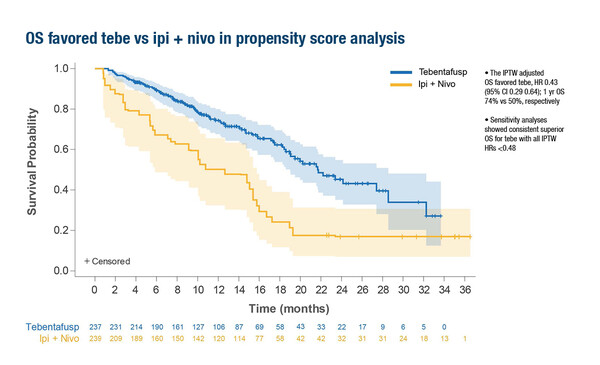
A second study presented at the Congress provided a propensity-score weighted comparison of tebentafusp or pembrolizumab versus combination ipilimumab/nivolumab in patients with untreated mUM, based on the 202 Study and the GEM-1402 trial of ipilimumab/nivolumab (Abstract 823P). The inverse probability of treatment weight (IPTW)-adjusted OS favoured tebentafusp over ipilimumab/nivolumab (hazard ratio [HR] 0.43; 95% CI 0.29–0.64; 1-year OS 74% versus 50%, respectively)
Bol considers these data to be useful in the absence of a randomised phase III trial. “The comparison of tebentafusp versus ipilimumab/nivolumab is particularly interesting because ipilimumab/nivolumab appears to achieve a higher OS response rate than anti-PD-1 alone,” she says. “Tebentafusp seems to be favourable over ipilimumab/nivolumab, which is unexpected based on the low response rates with tebentafusp. In the GEM-1402 study the response rates with ipilimumab/nivolumab are slightly higher than we see with tebentafusp so you would expect ipilimumab/nivolumab to do better, but tebentafusp appears better based on this analysis. This is quite interesting as normally you would consider response to be the most important predictor of long survival.”
Results from a third study, the single-arm phase II IMCgp100-102 trial (102 Study), revealed that the strongest baseline predictors of long-term (≥2-years) OS in patients with mUM receiving tebentafusp therapy were low serum lactate dehydrogenase, alkaline phosphatase and circulating tumour (ct)DNA and, in tumours, a low M2 macrophage:CD3+ T-cell ratio (Abstract 843P). The results also suggested that ctDNA reduction or clearance may be a better early surrogate of long-term OS than radiographic response. Commenting on these results, Bol warns that, “We do not know if these baseline characteristics are predictors of treatment response or are just prognostic factors as these data are based on patients with mUM in the 102 Study, all of whom received tebentafusp.” Bol would be interested to see the same analysis conducted for the randomised 202 Study in patients with previously untreated mUM to examine whether these are prognostic factors only or if they are predictive of treatment response to tebentafusp. She thinks that logically, a reduction in ctDNA would correlate with response and long-term OS, but stresses that, as tebentafusp is currently the only licensed treatment for this patient group, data for baseline predictors of treatment response may not be useful in determining treatment choices.
In conclusion, tebentafusp has provided the first specific treatment with survival benefit for patients with HLA-A*02:01-positive mUM.
Abstracts presented:
Sato T, et al. Long-term survivors on tebentafusp in phase 2 trial of previously treated patients with metastatic uveal melanoma. ESMO Congress 2022, Abstract 843P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Piulats Rodriguez JM, et al. A propensity score weighted comparison of tebentafusp or pembrolizumab versus combination ipilimumab and nivolumab in untreated metastatic uveal melanoma. ESMO Congress 2022, Abstract 823P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Koch EC, et al. Outcomes of immune checkpoint inhibitors in patients with metastatic uveal melanoma treated with tebentafusp. ESMO Congress 2022, Abstract 831P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform