Studies also suggest hope for TKI inhibitor combinations in patients with symptomatic brain metastases
Very encouraging data in the management of melanoma brain metastases – both asymptomatic and symptomatic – have been reported at the ESMO Congress 2021.
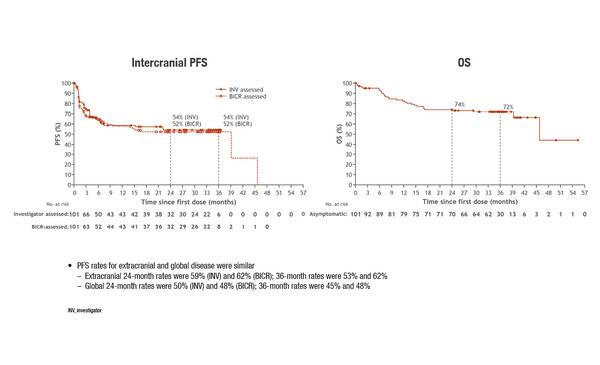
In three-year results presented today from the CheckMate 204 study, ipilimumab/nivolumab was associated with an investigator-assessed intracranial progression-free survival (icPFS) rate of 54% and an overall survival (OS) rate of 72% among 101 patients with asymptomatic brain metastases, at a minimum follow-up of 34 months (Abstract 1039MO). In 18 patients with symptomatic brain metastases, 36-month icPFS was 19% and OS was 37%. The first results of the blinded independent central review showed high concordance with investigator-assessed responses in patients with both asymptomatic and symptomatic metastases.
“Brain metastases are a huge problem in melanoma and affect a large proportion of patients, so we really need better therapies to manage them,” highlights Prof. Olivier Michielin from the Department of Oncology, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, commenting on these results. “In CheckMate 204, the durability of the icPFS rates in asymptomatic patients is a highly significant finding in terms of clinical management,” he says. “It is very reassuring to note that the rates are comparable to those reported for the ipilimumab/nivolumab arm of the ABC trial (54% CheckMate 204 and 46% ABC) (J Clin Oncol 2021;39(15 Suppl):Abstract 9508). The long-term data from CheckMate 204 support ESMO Guideline recommendations (Ann Oncol 2019;30:1884-1901) and confirm that this immunotherapy combination should be the standard choice of treatment for patients with melanoma and asymptomatic brain metastases.” However, according to the expert, the optimal treatment scheduling still needs to be identified, and the ABC-X trial is currently investigating ways to combine ipilimumab/nivolumab and stereotactic radiotherapy (J Clin Oncol 2019;37(15 Suppl):Abstract TPS9600).
A second study confirms activity of targeted therapy that has been suggested by other combinations, such as trametinib/dabrafenib.
Preliminary results from the GEM1802/EBRAIN-MEL phase II trial reported intracranial response rates (icRRs) of 64.3% and 63.6% in asymptomatic and symptomatic patients, respectively, with the tyrosine kinase inhibitors (TKIs) encorafenib plus binimetinib followed by radiotherapy in 25 patients with BRAF-mutated melanoma-associated brain metastases (Abstract 1038MO). The corresponding 6-month icPFS rates were 70.1% and 64.3%.
“This new TKI combination shows remarkable activity – 64% icRR – in patients with asymptomatic metastases, and the data look extremely promising. However, the issue is the duration of benefit and it is too soon to tell this from these early, preliminary findings,” Michielin cautions.
In terms of patients with asymptomatic brain metastases, the data from these two trials show important differences. “The benefit of ipilimumab/nivolumab is much lower in symptomatic patients than in asymptomatic patients, whereas the activity of the TKI combination was similar in both patient groups,” says Michielin. “The difference is in part due to the fact that TKI activity is tumour-cell centric, so these agents work independently of the tumour immune microenvironment, unlike immunotherapy, which is affected by corticosteroid usage for symptom control. The results from GEM1802/EBRAIN-MEL are promising for TKI use in melanoma brain metastases, particularly for symptomatic patients, but we need more patients and much longer follow-up,” he concludes.
Marquez-Rodas I et al. Intracranial activity of encorafenib and binimetinib followed by radiotherapy in patients with BRAF mutated melanoma and brain metastasis: preliminary results of the GEM1802/EBRAIN-MEL phase II clinical trial. ESMO Congress 2021, Abstract 1038MO
Mini oral session – Melanoma and other skin tumours 20.9.2021, h. 17:30 – 17:35, Channel 2
Margolin KA et al. CheckMate 204: 3-year outcomes of treatment with combination nivolumab (NIVO) plus ipilimumab (IPI) for patients (pts) with active melanoma brain metastases (MBM). ESMO Congress 2021, Abstract 1039MO
Mini oral session – Melanoma and other skin tumours 20.9.2021, h. 17:35 – 17:40, Channel 2