A significant progression-free survival improvement after re-treatment with olaparib was reported in the OReO/ENGOT Ov-38 trial
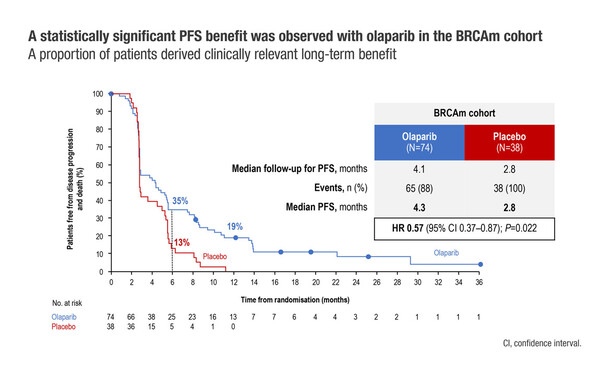
Late-Breaking results from the phase III OReO/ENGOT Ov-38 trial (LBA33) demonstrated that re-treatment with olaparib significantly prolonged progression-free survival (PFS) compared with placebo in both BRCA1/2-mutated (4.3 months versus 2.8 months, respectively, hazard ratio [HR] 0.57; p=0.022) and non-BRCA1/2-mutated disease (5.3 months versus 2.8 months, respectively, HR 0.43; p=0.002).
More and more patients with epithelial ovarian cancer will receive a PARP inhibitor as upfront therapy following positive results in the SOLO1, PAOLA-1 and the PRIMA/ENGOT-OV26/GOG-3012 studies in which olaparib prolonged the time to recurrence in BRCA1/2-mutated disease and niraparib prolonged progression-free survival time, regardless of homologous-recombination status. However, conserving platinum sensitivity in patients with disease progression on or after PARP inhibitor maintenance remains an unresolved issue. OReO/ENGOT Ov-38 is the first phase III study to evaluate PARP inhibitor maintenance re-treatment in this setting, and the findings will undoubtedly help inform the most appropriate strategy.
In the OReO/ENGOT Ov-38 study, patients with platinum-sensitive, non-mucinous epithelial ovarian cancer and one prior line of PARP inhibitor maintenance therapy who were responsive to their most recent platinum-based chemotherapy were randomised 2:1 to olaparib (300 mg bid [or 250 mg bid if 300 mg not previously tolerated]) or placebo until disease progression. Of 220 patients enrolled, 112 had BRCA1/2-mutated disease and 108 had non-BRCA1/2-mutated disease. Patients were heavily pre-treated, with most patients in both the BRCA1/2-mutated (93%) and non-BRCA1/2-mutated (86%) arms having received ≥3 prior lines of chemotherapy. The median duration of prior PARP inhibitor therapy was longer for patients with BRCA1/2-mutated disease (18.3–21.2 months) than those with non-BRCA1/2-mutated disease (12.4–12.6 months).
Among patients with BRCA1/2-mutated ovarian cancer, 6-month PFS rates were 35% with olaparib and 13% with placebo, while at 12 months, the respective rates were 19% and 0%. In the non-BRCA1/2-mutated cohort, respective PFS rates with olaparib and placebo were 30% and 7% at 6 months, and 14% and 0% at 12 months.
Adverse events (AEs) were more common with olaparib than placebo. Among patients with BRCA1/2-mutated disease, grade ≥3 AEs occurred in 15% of olaparib-treated versus 5% of placebo-treated patients, while among non-BRCA1/2-mutated patients, these AEs occurred in 21% of olaparib-treated versus 8% of placebo-treated patients. Treatment discontinuation because of an AE also occurred more frequently in olaparib-treated patients (3% with BRCA1/2-mutated disease and 1% with non-BRCA1/2-mutated disease) than in placebo-treated patients (0% of patients).
Pujade-Lauraine E. Maintenance olaparib rechallenge in patients (pts) with ovarian carcinoma (OC) previously treated with a PARP inhibitor (PARPi): Phase IIIb OReO/ENGOT Ov-38 trial. ESMO Congress 2021, Abstract LBA33
Proffered Paper session – Gynaecological cancers 17.9.2021, h. 14:20 – 14:30, Channel 3