Favourable results were presented from three phase III trials which are likely to lead to a practice-changing role for PARP inhibitors in the treatment of newly diagnosed advanced ovarian cancer
At yesterday’s Presidential Symposium, favourable results were presented from three phase III trials, which are likely to lead to a practice-changing role for PARP inhibitors in the treatment of newly diagnosed advanced ovarian cancer. PARP inhibitors have started to reshape the treatment landscape in ovarian cancer with results from the SOLO1 trial presented at the ESMO Congress last year, which reported that maintenance therapy with olaparib improved progression-free survival (PFS) in women with newly diagnosed high-grade advanced ovarian cancer and a BRCA1/2 mutation.
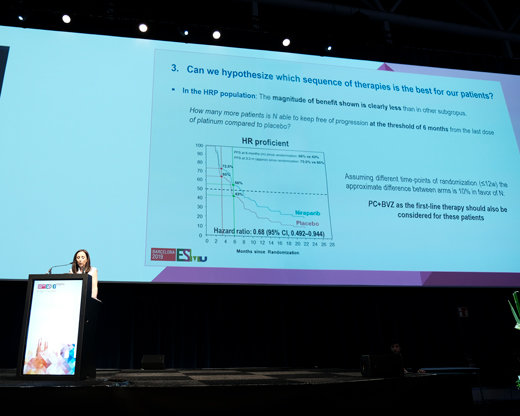
One of the important questions that late-breaking trials are seeking to investigate is whether PARP inhibitors only benefit those with BRCA mutations or if they could be used to treat patients positive for homologous recombination deficiency (HRD), or have an even wider use in all patients with newly diagnosed advanced ovarian cancer, including in combination with chemotherapy or bevacizumab. The PRIMA/ENGOT-OV26/GOG-3012 trial revealed that maintenance niraparib following platinum-based chemotherapy substantially extended PFS compared with placebo in the overall population (median 13.8 months versus 8.2 months; hazard ratio [HR] 0.62; 95% confidence interval [CI] 0.50–0.75; p<0.0001) (Abstract LBA1). Among the 51% of the 733 patients enrolled who were positive for HRD, there was more than a doubling in the duration of PFS with maintenance niraparib (median 21.9 months versus 10.4 months; HR 0.43; 95% CI 0.31–0.59; p<0.0001).
Initiating PARP inhibition earlier in first-line treatment was studied in the PAOLA-1/ENGOT-OV25 trial, which investigated adding olaparib to bevacizumab as maintenance treatment after platinum-based chemotherapy (Abstract LBA2_PR). Olaparib plus bevacizumab significantly improved PFS compared with placebo plus bevacizumab in the overall population (median 22.1 months versus 16.6 months; HR 0.59; 0.49–0.72; p<0.0001), regardless of BRCA mutation status. In patients with BRCA-mutated tumours, olaparib plus bevacizumab was associated with improved PFS (median 37.2 months versus 21.7 months; HR 0.31; 0.20–0.47), with less benefit in patients with non-BRCA-mutated tumours (median 18.9 months versus 16.0 months; HR 0.71; 0.58–0.88). There appeared to be no substantial benefit for olaparib plus bevacizumab maintenance in those with negative or unknown HRD status (median PFS 16.9 months versus 16.0 months; HR 0.92; 0.72–1.17).
Also studying upfront PARP inhibition without selection according to BRCA mutation status, the VELIA/GOG-3005 trial is the first phase III trial to investigate the addition of veliparib to front-line paclitaxel/carboplatin with or without veliparib maintenance (Abstract LBA3). Of the 1,140 patients enrolled, 26% had a BRCA mutation. Veliparib added to front-line chemotherapy and continued as monotherapy maintenance significantly extended PFS across the entire population compared with chemotherapy plus placebo maintenance (median 23.5 months versus 17.3 months; HR 0.68; 95% CI 0.58–0.83; p<0.001), with seemingly greater benefits seen in those with a BRCA mutation (median 34.7 months versus 22.0 months; HR 0.44; 95% CI 0.28–0.38; p<0.001).
"We now have three phase III trials reporting meaningful improvements in PFS with the addition of PARP inhibitors to first-line therapy in ovarian cancer patients beyond those with a BRCA mutation—extending the population of women who could benefit from PARP inhibitors. The greatest magnitude of improvement is seen in patients with a BRCA mutation,” comments Dr Susana Banerjee from The Royal Marsden NHS Foundation Trust and Institute of Cancer Research, London, UK. "These trials prompt many other interesting questions: what is the best first-line PARP inhibitor treatment strategy? For example, can some patients receive PARP inhibitor maintenance alone or is a combination with bevacizumab, for instance, better? How is it best to select patients for PARP inhibitors—are current HRD tests ready for clinical practice? Is there a place for PARP inhibitors in less advanced ovarian cancer? Clinical trials are needed to address these questions.”
No major surprises were evident from toxicity data presented yesterday. It is important to evaluate dose reductions, dose interruptions and drug discontinuations, according to Banerjee. "We need to understand how best to manage toxicities, and support patients so women can maintain a good quality of life on maintenance therapy.” She concludes, "Since last year’s practice-changing SOLO1 trial was presented at ESMO, olaparib maintenance therapy has become standard of care for women with newly diagnosed ovarian cancer and a BRCA mutation. These three phase III trials presented at ESMO Congress 2019 are incredibly important and are highly likely to shape clinical practice internationally for newly diagnosed ovarian cancer patients with and without a BRCA mutation. Ongoing phase III, first-line trials will help address whether PARP inhibitors in combination with immunotherapy can further improve outcomes in ovarian cancer. The ultimate goal is to have more long-term survivors and more women cured from this devastating disease.”
ESMO Congress 2019 abstracts:
- LBA1 - Niraparib therapy in patients with newly diagnosed advanced ovarian cancer (PRIMA/ENGOT-OV26/GOG-3012 study)
- LBA2_PR - Phase III PAOLA-1/ENGOT-ov25 trial: Olaparib plus bevacizumab (bev) as maintenance therapy in patients (pts) with newly diagnosed, advanced ovarian cancer (OC) treated with platinum-based chemotherapy (PCh) plus bev
- LBA3 - VELIA/GOG-3005: Integration of veliparib (V) with front-line chemotherapy and maintenance in women with high-grade serous carcinoma of ovarian, fallopian tube, or primary peritoneal origin (HGSC)