Analysis from MARIPOSA-2 and FLAURA2 trials show a potential change of treatment paradigm in both first- and second-line settings
EGFR mutations in non-small-cell lung cancer (NSCLC) are twice as common among Asian patients compared with Caucasian patients (Oncotarget. 2016;7:78985–78993) and pose a significant management challenge. Insights into recent treatment options were provided by two presentations at the ESMO Asia Congress 2023 (Singapore, 1–3 December).
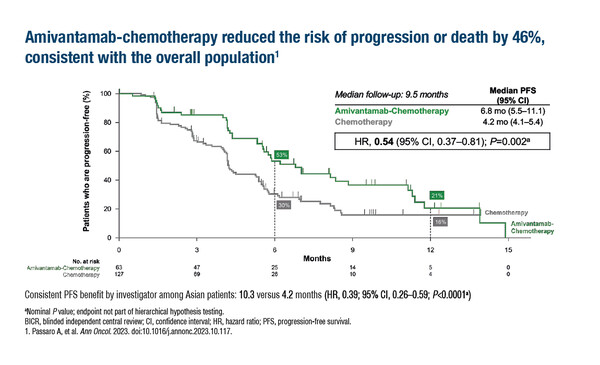
A subgroup analysis of Asian patients in the MARIPOSA-2 trial, presented at the ESMO Congress this year (Ann Oncol. 2023;34(Suppl_2):S1254–S1335), support the main trial analysis showing a significant improvement in progression-free survival (PFS) with the EGFR-MET bispecific antibody amivantamab plus chemotherapy compared with chemotherapy alone in patients with EGFR-mutated NSCLC progressing on osimertinib (LBA11). At a median follow-up of 9.5 months, amivantamab plus chemotherapy led to a 46% reduction in the risk of progression or death (hazard ratio [HR] 0.54; 95% confidence interval [CI] 0.37–0.81; p=0.002), with a median PFS of 6.8 months compared with 4.2 months for chemotherapy alone. The objective response rate was more than doubled with amivantamab plus chemotherapy (66% versus 32%; odds ratio 4.0; p<0.0001). The median intracranial PFS was 12.5 months with amivantamab plus chemotherapy versus 8.5 months with chemotherapy (HR 0.58; 95% CI 0.34–1.00; p=0.049). The amivantamab-plus-chemotherapy arm was associated with higher rates of neutropenia and thrombocytopenia, which occurred early, with low rates of febrile neutropenia and bleeding complications.
In another presentation, preliminary data from an exploratory analysis of the FLAURA2 trial, presented earlier this year (J Thorac Oncol 2023;18_Suppl:S36–S37), suggested that the addition of platinum-based chemotherapy to first-line osimertinib does not impact on acquired resistance mechanisms and should not influence targeted second-line treatment options (Abstract 514MO). Resistance mechanisms were broadly similar with osimertinib plus chemotherapy (n=53) and osimertinib alone (n=73), according to paired plasma samples from patients with EGFR-mutated circulating tumour DNA samples at baseline and disease progression. However, numerically fewer patients receiving osimertinib plus chemotherapy had acquired EGFR C797S (2 versus 9 receiving osimertinib monotherapy) and MET amplification (5 versus 10). No novel resistance mechanisms were detected specifically in patients receiving osimertinib plus chemotherapy.
According to Dr Antonio Passaro from the European Institute of Oncology, Milan, Italy, “Based on the ground-breaking results presented this year, the therapeutic landscape of EGFR-mutated NSCLC is on the verge of a significant transformation, introducing several innovative and improved therapeutic options in both first- and second-line settings.” He continues: “While combinations of multiple therapeutic agents have come with increased rates of toxicity, these have not been shown to be dose limiting, and low discontinuation rates have been maintained in all these studies. With proper management and proactive, dedicated treatment, the use of new therapeutic agents within these novel combinations cannot be ignored as effective therapeutic strategies.”
Passaro concludes by looking at the challenges ahead in EGFR-mutated NSCLC: “The main issue today lies in making comprehensive molecular analysis – including next-generation sequencing – accessible to all patients diagnosed with early or advanced stages of the disease. While discussing testing might seem obvious, far too many patients worldwide still do not receive proper and extensive molecular analysis, which ensures access to targeted and effective therapies. We also need to provide timely access to new therapeutic strategies, a challenge that is compounded by therapeutic disparities across different nations and continents.”
Abstracts discussed:
Shih J-Y, et al. Amivantamab plus chemotherapy vs chemotherapy among Asian patients with EGFR-mutant advanced NSCLC after progression on osimertinib: A MARIPOSA-2 subgroup analysis. ESMO Asia Congress 2023, LBA11
Proffered Paper Session: Thoracic Cancer, 01.12.2023, h. 10:45 – 11:55 SGT, Hall 406
Lee CK, et al. Acquired mechanisms of resistance to first-line (1L) osimertinib with or without platinum-based chemotherapy (CT) in EGFR-mutated (EGFRm) advanced NSCLC: Preliminary data from FLAURA2. ESMO Asia Congress 2023, Abstract 514MO
Mini Oral Session 2: Thoracic Cancer, 03.12.2023, h. 09:00 – 10:30 SGT, Hall 406