Encouraging EFS and OS results were presented at an ESMO Virtual Plenary for the use of the immune checkpoint inhibitor prior and after surgery
Perioperative use of the anti-PD-1 monoclonal antibody tislelizumab improves responses at the time of surgery and reduce the risk of disease recurrence in patients with early-stage resectable non-small cell lung cancer (NSCLC), as confirmed by updated results from the RATIONALE-315 (NCT04379635) presented at the ESMO Virtual Plenary in February 2024.
Although surgery is the most efficient frontline therapy for this tumour type, recurrence rates at 5-years are still unacceptably high, raising the need for more effective neoadjuvant and adjuvant strategies to improve the patients’ outcomes. In the Phase III RATIONALE-315 trial, the efficacy of tislelizumab prior and after surgery was assessed in 453 patients with treatment-naïve resectable stage II–IIIA NSCLC. Patients were randomised to receive either tislelizumab combined with platinum-doublet chemotherapy or a placebo plus chemotherapy in the neoadjuvant setting, and to receive tislelizumab monotherapy or a placebo as adjuvant treatment.
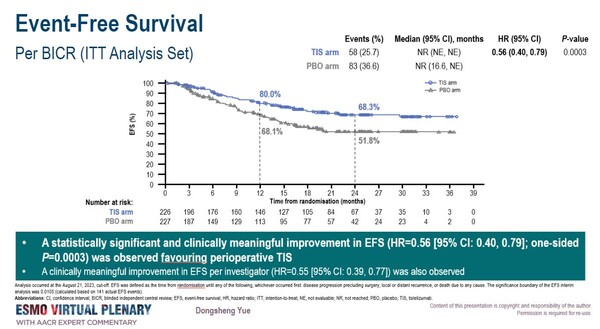
In the interim analysis, a clinically meaningful and statistically significant benefit in event-free survival (EFS) by blinded independent central review, the primary endpoint, in the tislelizumab group was observed at a median follow-up of 22 month (HR=0.56 [95% CI: 0.40, 0.79]; one-sided P=0.0003), and it was generally consistent across all predefined subgroups. The neoadjuvant combination therapy showed a statistically significant improvement in the second primary endpoint, major pathological response (MPR), with a difference of 41.1% between the two groups (95% CI: 33.2, 49.1; P<0.0001).
Encouraging data were also presented for the secondary endpoint, with overall survival (OS) benefit trend favouring perioperative tislelizumab (HR=0.62 [95% CI: 0.39, 0.98]; one-sided P=0.0193) and 40.7% of patients treated with the tislelizumab combination achieving pathological complete response (pCR) compared to 5.7% of patients in the chemotherapy plus placebo arm (35.0%; 95% CI: 27.9, 42.1; P<0.0001).
“The data presented at the ESMO Virtual Plenary confirms the role of tislelizumab as a perioperative strategy for patients with early-stage resectable NSCLC. The benefit in EFS was aligned even in magnitude with that of other PD-1 inhibitors”, says Luis Paz-Ares, Hospital Universitario 12 de Octubre, Madrid, Spain commenting on the study results which are consistent with those from a first interim analysis presented at the ESMO Congress 2023 (Ann Oncol. Volume 34, Supplement 2, S1299, Oct 2023). “The PD-1-based regimen could be considered as a novel standard option of care, particularly in Asia, but validation in Western patients would be welcome in the future.”
Abstract discussed
D. Yue et al. RATIONALE-315: Event-Free Survival (EFS) and Overall Survival (OS) of Neoadjuvant Tislelizumab (TIS) plus Chemotherapy (CT) with Adjuvant TIS in Resectable Non-Small Cell Lung Cancer (NSCLC). (VP1-2024). ESMO Virtual Plenary, 15 February 2024.