Data from two studies suggest a good rationale for targeting T-cell dysfunction with precision immunotherapy to intercept pre-cancerous lesions
Detectable pre-malignant changes represent lesions that have escaped the immune system, and targeting interventions to prevent or intercept pre-malignant lesions before significant immune dysregulation has occurred is being investigated as a possible immune-based strategy (J Immunother Cancer. 2025;13:e010419). However, knowledge of actionable pathways relating to tumour-specific T-cell dysfunction in pre-cancer is relatively limited, notes Dr Krijn Dijkstra from the Netherlands Cancer Institute, Amsterdam, Netherlands. “What is still unclear is to what extent such pre-invasive lesions are recognised by T cells, and if they are, what are the mechanisms that disable T cells? Are they different from those in advanced cancers?” Two presentations at the Moluecular Analysis for Precision Oncology (MAP) Congress 2025 (Paris, 15–16 September) address these questions to determine whether there is a good rationale for immune interception.
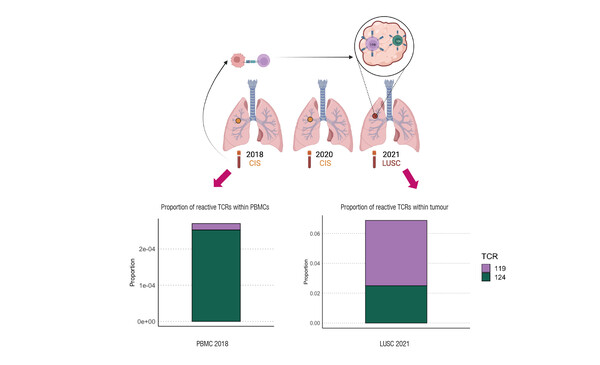
The first analysis investigated antigen-specific T cells in smokers undergoing autofluorescence bronchoscopy surveillance for lung cancer progression (Abstract 22MO). “What is quite striking is that clear signs of T-cell recognition and reactivity are observed in these early carcinoma in-situ (CIS) airway lesions, and the T cells are adopting an exhausted phenotype, so there is something driving them to exhaustion prior to the onset of invasive disease, and that is what we need to target,” notes Dijkstra. “That active recognition by T cells is observed suggests that immune interception may be possible.”
In addition, T cells in peripheral blood were found to have a specific signature associated with high-grade pre-invasive disease, indicating that this may be a biomarker that could be used to identify higher risk patients. Dijkstra emphasises the need for biomarkers to understand which pre-invasive lesions should be targeted: “We would only want to intervene in high-risk subgroups as we have to balance the potential adverse effects with the potential benefits of immune interception. Some lesions will spontaneously regress or remain stable, so only those that are most likely to develop into a cancer should be targeted.”
A second presentation reported T-cell subset composition and associated transcriptomic changes across healthy, pre-malignant, and a range of tumour tissues (a ‘pan-tissue atlas’ of T cells) to look for common patterns and determine the extent to which early lesions are capable of reorganising their microenvironment into an immunosuppressive state (Abstract 178O).
The results showed that the pre-cancer T-cell landscape differed markedly from that of normal tissue, suggesting that a distinct immunosuppressive microenvironment was already established in the pre-malignant stage, characterised by more regulatory and exhausted T cells than healthy samples. In addition, more progenitor exhausted T cells were observed in pre-malignant samples than in advanced cancers. “According to recent research, this subtype of exhausted T cell is thought to be relatively sensitive to immune checkpoint inhibition (Clin Transl Med. 2024;14:e1817). This suggests that immune interception may be a promising therapeutic approach,” remarks Dijkstra. In the study, one potential checkpoint inhibitor target, glucocorticoid-induced TNFR-related protein (GITR), was identified across a number of different pre-cancerous tissues and may be a rational target for immune interception.
An important next step will be to translate these descriptive findings into functional models. “The identification of a pan-tissue target is eagerly awaited, but it will be crucial to validate the current descriptive findings in functional preclinical models,” he concludes.
Programme details
Abstract 22MO - Rogers A, et al. Exploiting neoantigen reactive CD8 T cell dysfunction to intercept lung squamous carcinogenesis. MAP Congress 2025
Mini Oral Session, 16.09.2025, h. 13:00 - 14:00, Auditorium Amphi Lovoisier
Abstract 178O - Ahlmann-Eltze C, et al. A pan-tissue atlas of T cells during tumorigenesis reveals pre-cancer interception targets. MAP Congress 2025
Proffered Paper Session, 15.09.2025, h. 14:15 - 15:45, Auditorium Amphi Lovoisier