AKT and EZH2 are identified as potential therapeutic targets to limit acquired resistance to osimertinib in patients with EGFR-mutated tumours of the lung
A Mini Oral session at the Molecular Analysis for Precision Oncology Virtual Congress 2021 provides the first comprehensive molecular characterisation of phenotypic transformation of adenocarcinoma of the lung (LUAD) to squamous cell carcinoma (LUSC), a phenomenon that often leads to acquired resistance to targeted treatments (Abstract 1MO).
“Adenosquamous tumours have been associated with a significantly poorer prognosis than adenocarcinoma or squamous cell tumours, suggesting the need for new therapeutic approaches,” explains the study first author, Dr Álvaro Quintanal-Villalonga from the Memorial Sloan Kettering Cancer Center, New York, USA. “However, in order to identify potential therapeutic approaches for squamous transdifferentiation, we first need to understand the biology behind it.”
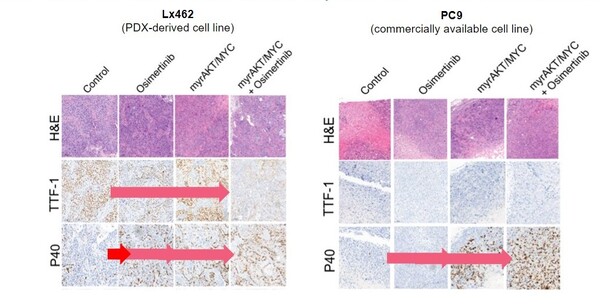
In the study, the researchers performed microdissection of the adenocarcinoma and squamous components in adenosquamous tumours, they then conducted molecular analyses on each component separately to overcome issues related to the accessibility to pre- and post-transdifferentiation tumour tissue. Additionally, some of the few pre- and post-transdifferentiation cases that they were able to access were analysed. Molecular characterisation was performed using detailed genomic (whole exome sequencing [WES]), epigenomic (bisulphite sequencing), transcriptomic (RNAseq) and proteomic (antibody array) profiling (Figure). Further validation of the findings was achieved using preclinical models.
The study identified that mutations in TBX3, MET and RBM10 were enriched specifically in transforming adenocarcinoma, suggesting they may help identify adenocarcinoma tumours at high risk of transitioning to a squamous phenotype. Additionally, three potential drivers or mediators of squamous transdifferentiation were identified: the stemness-related transcription factor MYC, the AKT signalling pathway, and the epigenetic remodelling complex PRC2, previously involved in lineage plasticity.
Combined overexpression of MYC and AKT in preclinical adenocarcinoma models induced a squamous-like phenotype, and EZH2, the catalytic component of the PRC2 complex, was upregulated upon transdifferentiation. Notably, the EZH2 inhibitor, ORS1 was able to prevent squamous relapse occurring upon osimertinib treatment in a patient-derived xenograft model of adeno-to-squamous carcinoma transdifferentiation, and both ORS1 and the AKT inhibitor, samotolisib, were able to re-sensitise tumours to osimertinib after squamous transdifferentiation had occurred.
Transformation of LUAD to LUSC occurs with an up to 9% incidence in EGFR-mutant tumours relapsed on osimertinib (Clin Cancer Res. 2020;26:2654–2663). “By translating our findings to the clinic and combining osimertinib with pharmacological targeting of EZH2 and AKT signalling, we may be able to help prevent and treat this adverse transition,” concludes Quintanal-Villalonga. Currently, there are no further clear-cut therapeutic options other than chemotherapy and locally ablative therapy to offer to patients relapsing after the EGFR inhibitor. “The study highlights the value of multi-omic analyses to understand complex clinical phenomena. This dataset will soon be available as a resource from which we hope other researchers in the field will benefit.”