Study confirms favourable safety profiles and encouraging efficacy of an anti-claudin 18.2 antibody-drug conjugate
Updated safety and efficacy data of IBI343, an anti-claudin 18.2 (CLDN18.2) antibody-drug conjugate, show promise in patients with advanced pancreatic ductal adenocarcinoma (PDAC) and positive CLDN18.2 expression (defined as ≥ 60% intensity of 1+/2+/3+ staining cells by IHC) who failed or were intolerant to standard treatment, as reported in a presentation at the ESMO Asia Congress 2024 (Singapore, 6-8 December) (Abstract 132MO).
Targeting claudins represents a significant advance in the pursuit of precision oncology, and some early-phase studies have recently shown encouraging results. IBI343 is an antibody-drug conjugate (ADC) consisting of anti-CLDN18.2 monoclonal antibody conjugated to exatecan, a topoisomerase I inhibitor. Preliminary results from a phase 1a/b, dose escalation, expansion, and extension study (NCT05458219) were presented earlier this year showing favorable safety profiles and encouraging efficacy of the anti-claudin treatment in patients with CLDN18.2-positive PDAC and biliary tract cancer (BTC) (J Clin Oncol 42, 3037-3037; 2024).
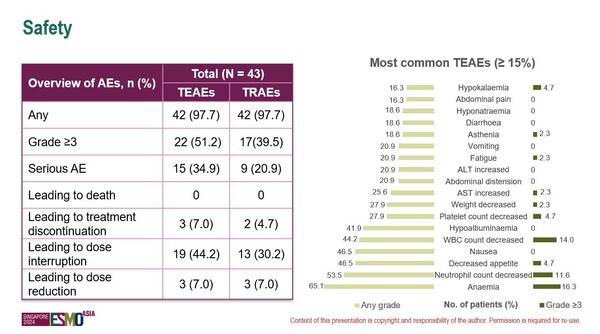
Updated data from the dose expansion cohort of the study, discussed in Singapore, involving 43 patients with CLDN18.2-positive PDAC as of data cutoff (September 6, 2024) receiving intravenous IBI343 at 6 mg/kg once every three weeks (Q3W), show no new safety signal compared to previous reports (Figure). Treatment emergent adverse events (TEAEs) occurred in 97.7% of patients, including ≥G3 TEAEs in 22 patients. Most common TEAEs were anaemia, decreased neutrophil count, decreased appetite, nausea, decreased white blood cell count and hypoalbuminemia. TEAEs led to treatment discontinuation in 3 patients, and none led to death.
Regarding secondary endpoints, objective response rate (ORR) and disease control rate (DCR) were 23.3% (95% CI, 11.8-38.6) and 81.4% (95% CI, 66.6-91.6) respectively, and progression-free survival (PFS) was 5.3 months (95% CI, 4.1-7.4) with a median follow-up of 5.7 months.
Programme details
Yu X, et al. Anti-claudin18.2 (CLDN18.2) antibody-drug conjugate (ADC) IBI343 in patients (pts) with advanced pancreatic ductal adenocarcinoma (PDAC): updated results from a phase 1 study. ESMO Asia Congress 2024, Abstract 132MO
Mini Oral Session – Gastrointestinal tumours, 07.12.2024, h. 09:00 – 10:40, Hall 404