Alternatives to osimertinib for first-line treatment of EGFR-mutated NSCLC are explored among Asian populations
Trial results for two new third-generation EGFR tyrosine kinase inhibitors (TKIs) presented at the European Lung Cancer Congress (ELCC) 2022 indicate efficacy close to that of osimertinib in Chinese patients with non-small-cell lung cancer (NSCLC) and EGFR mutations, with data that may show differentiation awaited.
“In China, around half of patients with NSCLC have EGFR mutations (Oncologist. 2019;24:e1070–e1081),” says Prof. Benjamin Besse from the Institut Gustave Roussy, Villejuif, France. “Even though effective EGFR TKIs are approved, we still need new strategies to overcome resistance, to increase duration of response and improve safety, since some patients have contraindications to currently available agents.”
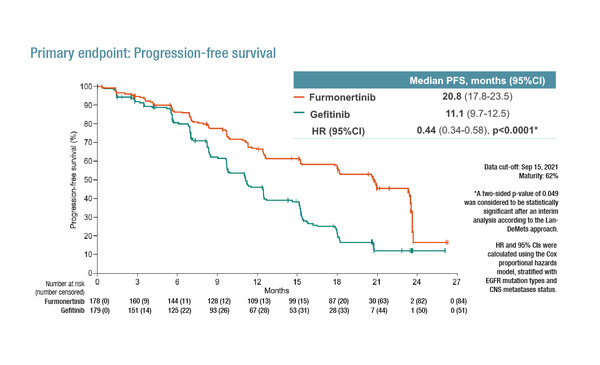
As presented in a Proffered Paper Session, the phase III FURLONG trial investigated furmonertinib (AST2818) versus gefitinib in 358 Chinese patients with treatment-naïve stage IIIB/IIIC/IV NSCLC and a sensitive EGFR mutation (exon19del or L858R) (Abstract 1O). After a median follow-up of 21.0 months, the primary endpoint of progression-free survival (PFS) was significantly longer with furmonertinib than gefitinib (median 20.8 versus 11.1 months; hazard ratio [HR] 0.44; 95% confidence interval [CI] 0.34–0.58; p<0.0001). Median duration of exposure was 18.3 months in the furmonertinib group and 11.2 months in the gefitinib group, with grade ≥3 treatment-related adverse events experienced by 11% and 18% of patients, respectively.
“It would have been interesting to use osimertinib rather than gefitinib as the control arm since osimertinib demonstrated extended survival compared with first-generation TKIs, with a similar safety profile (N Engl J Med. 2020;382:41–50),” comments Besse. “When we look at results from FURLONG with furmonertinib compared with results from FLAURA China with osimertinib (Target Oncol. 2021;16:165–176), it appears to be no major differences regarding efficacy for furmonertinib, although data on overall survival (OS) and the rate of crossover will be important to consider when available.” Besse is also keen to assess safety data with furmonertinib more carefully to determine if there are any advantages, such as reduced frequency of anaemia and QT prolongation, compared with osimertinib. In addition, verifying if the degree of different activity observed with osimertinib in patients with EGFR exon 19 and 21 mutations is replicated in FURLONG could be informative.
In a Mini Oral Session at ELCC 2022, results from a phase II trial of oritinib (SH-1028) in 227 previously treated patients with locally advanced or metastatic NSCLC and an EGFR T790M mutation were presented (Abstract 7MO). The objective response rate – the primary efficacy endpoint – was 60.4% (95% CI 53.7–66.8), the disease control rate was 92.5% (95% CI 88.3–95.6) and median PFS was 12.6 months (95% CI 9.7–15.3). The most common treatment-related adverse events (TRAEs) were diarrhoea (41.9%), increased blood creatine phosphokinase (23.8%) and decreased white blood cell count (13.2%). Grade ≥3 TRAEs included increased blood creatine phosphokinase (4.0%), diarrhoea (2.2%) and decreased lymphocyte count (1.8%).
“Findings with oritinib in EGFR T790M-positive NSCLC are similar to those seen with osimertinib in the AURA3 trial (N Engl J Med. 2017;376:629–640) and without mature OS data, it is difficult to conclude more than that oritinib is a ‘me-too’ drug,” says Besse, but he highlights that investigating alternative options of third-generation TKIs to osimertinib may be useful in the case of limited access to the agent or contraindications. Although osimertinib showed a less pronounced OS benefit among Asian patients and those with an EGFR exon 21 p.L858R mutation, an ESMO Expert Consensus paper on the management of EGFR-mutant NSCLC states that this should not restrict its use as first-line therapy for these patient subgroups (Ann Oncol. 2022;S0923-7534(22)00112-0).
Discussing both sets of results and the field in general, he notes, “Data on resistance acquired after upfront and second-line use of new third-generation TKIs will be interesting as it is not clear whether mechanisms will be the same as for available TKIs. Furthermore, now that fourth-generation EGFR TKIs are in development – agents that can target double mutations or even triple mutations – we may need to revisit the concept of sequencing and redefine our precision medicine pathways.”
Abstracts discussed:
Shi Y, et al. Furmonertinib versus gefitinib in treatment-naïve EGFR mutated non-small cell lung cancer: a randomized, double-blind, multi-center, phase III study (FURLONG). European Lung Cancer Congress 2022, Abstract 1O
Proffered Paper Session, 31.03.2022, h. 15:15 – 16:35, Congress Hall
Zhou C, et al. Oritinib (SH-1028), a third-generation EGFR tyrosine kinase inhibitor in locally advanced or metastatic NSCLC patients with positive EGFR T790M: results of a single-arm phase II trial. European Lung Cancer Congress 2022, Abstract 7MO
Mini Oral Session 2, 30.03.2022, h. 14:15 – 15:15, Club A