Overall survival data are in line with those from other MET tyrosine kinase inhibitors and show promise in a tumour subtype with poor prognosis
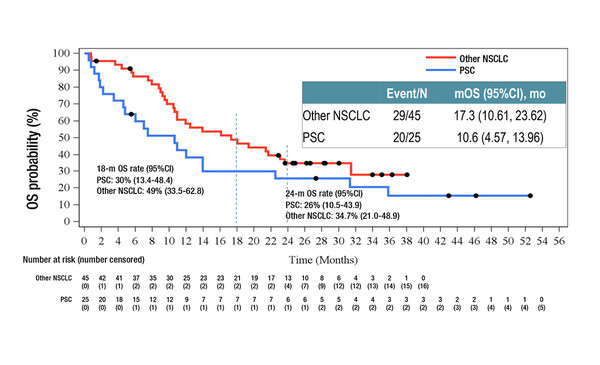
Final overall survival (OS) data from an open-label phase II trial revealed that, at a median follow-up of 28.4 months, savolitinib was associated with a median OS of 12.5 months (95% confidence intervals 10.5–21.4) in 70 Chinese patients with pre-treated (n=42) or treatment-naïve (n=28) pulmonary sarcomatoid carcinoma (PSC; n=25) or other non-small-cell lung cancers (NSCLCs) (n=45) harbouring MET exon14 skipping mutations (Abstract 2MO). The 18- and 24-month OS rates for all patients were 42% and 31%.
In subgroup analyses, the median OS times for pre-treated and treatment-naïve patients were 19.4 months and 10.9 months, respectively, with corresponding 18-month OS rates of 50% and 30%. Twelve PSC patients were pre-treated and 13 were treatment-naïve. The median OS times for PSC and other NSCLC patients were 10.6 months and 17.3 months, respectively, with corresponding 18-month OS rates of 30% and 49%. Among 15 patients with brain metastases, the median OS was 17.7 months the 18-month OS rate was 50%.
Grade ≥3 treatment-related adverse events were reported in 46% of patients, the most frequent being increases in aspartate aminotransferase (13%) and alanine aminotransferase (10%) along with peripheral oedema (9%). The incidence of adverse events was consistent across subgroups.
“We know that MET exon14 skipping mutations, which occur in 3–5% of patients with NSCLC, are driver mutations and that there is real clinical benefit in targeting them (Cancer Treat Rev. 2021;96:102179),” comments Dr Alfredo Addeo from Geneva University Hospital, Switzerland. “The activity of savolitinib in PSC, which has a poor prognosis, is encouraging and may help to define a group that could derive particular benefit from this treatment.” Also, this data support to perform molecular analysis on these cases to allow targeted treatments.
Although promising, however, Addeo cautions: “Data presented are not ground-breaking and are in line with what we have observed with the currently available MET tyrosine kinase inhibitors, tepotinib and capmatinib in MET exon14 skipping mutated NSCLC (N Engl J Med. 2020;383:931–943; N Engl J Med. 2020;383:944–957). We still urgently need new treatments that will help to raise the efficacy bar and provide more marked improvements in survival for what, in the case of PSC, is often a relatively young patient population.”
Abstract discussed:
Lu S, et al. Final OS results and subgroup analysis of savolitinib in patients with MET exon 14 skipping mutations (METex14+) NSCLC. European Lung Cancer Congress 2022, Abstract 2MO
Mini Oral Session 2, 30.03.2022, h. 14:15 - 15:15, Club A