Early-phase trials suggest that sitravatinib may reboot the tumour response to PD-1 inhibitors, but confirmation of efficacy is required and strategies are needed to adjust tolerability
Two poster presentations at the ESMO Targeted Anticancer Therapies Congress 2022 suggest encouraging findings from phase I and II trials in patients with non-small-cell lung cancer (NSCLC) treated with sitravatinib after progressing on anti-PD-(L)1-containing therapy.
“Treatment of immunotherapy-refractory NSCLC represents a real challenge,” says Prof. Martin Reck from the Lung Clinic Grosshansdorf, Airway Research Center North, German Center of Lung Research, Grosshansdorf, Germany. “Despite the investigation of multiple immunotherapy combinations, we have not seen convincing data showing benefits over standard docetaxel, and so we are really still at the starting point.”
Sitravatinib is a spectrum-selective inhibitor of tyrosine kinases – including TAM receptors and VEGFR2 – that are involved in the immunosuppression of the tumour microenvironment (TME) and that may mediate the development of resistance to checkpoint inhibitor (CPI) therapy. The hypothesis is that sitravatinib will re-establish the immunogenicity of the TME in patients with immunotherapy-refractory NSCLC and so enhance response to CPI therapy.
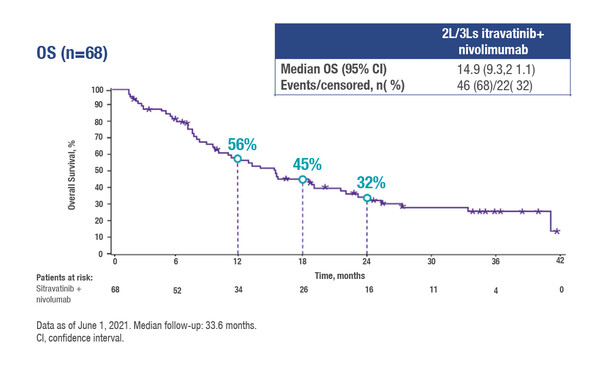
Updated results from the phase II MRTX-500 trial (NCT02954991) in a cohort of 68 patients with prior clinical benefit from CPI therapy show that the use of sitravatinib (120 mg once daily) plus nivolumab (every 2 or 4 weeks) as second- or third-line therapy was associated with a median overall survival (OS) of 15 months and a 1-year OS rate of 56% (Abstract 43P). The median follow-up was 34 months. The objective response rate (ORR) was 18% – with two of the patients achieving a complete response – and the median duration of response (DoR) was 13 months. Grade 3–4 treatment-related adverse events occurred in 66% of patients, the most common (≥16%) being hypertension and diarrhoea. Adverse event-related discontinuation rates for sitravatinib and nivolumab were 21% and 9%, respectively.
A phase Ib trial investigated the use of sitravatinib (120 mg daily) in combination with tislelizumab (200 mg every 3 weeks), an anti-PD-1 antibody designed to minimise the development of CPI resistance (Abstract 1P). Among 47 patients, with a median follow-up of 7.8 months, the confirmed ORR was 14%, the median DoR was 7 months and the median progression-free survival was 5 months. Grade ≥3 adverse events were seen in 68% of patients; the most common was hypertension (19%).
“The signals of clinical efficacy with sitravatinib plus CPI therapy combination are really encouraging in the heavily pre-treated immuno-oncology-refractory patients included in these early-phase trials,” says Reck. “It is interesting to note that the activity of tislelizumab as a combination partner was very similar to that of nivolumab, with ORRs of around 15% in both trials.” He continues, “Tolerability is going to be a key consideration in the use of sitravatinib in the clinic, with grade 3 or greater effects being observed in over 60% of patients in these trials. Grade 3 hypertension, a side effect consistent with the actions of sitravatinib at VEGFR2, has the potential to compromise treatment delivery and so modifications to dose or scheduling may be required. Furthermore, the use of innovative biomarkers to identify the patients who will benefit is of paramount importance. It is hoped that the results of the ongoing phase III SAPPHIRE trial (NCT03906071), which compares the combination of sitravatinib plus nivolumab to docetaxel in a randomised setting, will provide further insight into the clinical potential of this new treatment approach.”
Abstracts discussed:
Leal TA, et al. MRTX-500: Phase II trial of sitravatinib (sitra) + nivolumab (nivo) in patients (pts) with non-squamous (NSQ) non-small cell lung cancer (NSCLC) progressing on or after prior checkpoint inhibitor (CPI) therapy. ESMO Targeted Anticancer Therapies Congress 2022, Abstract 43P
Gao B, et al. Sitravatinib + Tislelizumab in Patients with Anti-PD-(L)1 Refractory/Resistant Metastatic Non-small Cell Lung Cancer (NSCLC). ESMO Targeted Anticancer Therapies Congress 2022, Abstract 1P