Blocking the tyrosine kinase cell-cycle progression regulator WEE1 may help to reinstate tumour immunogenicity and potentiate the effects of immune checkpoint inhibitors
The combination of the selective, small molecule WEE1 inhibitor adavosertib (AZD1775) and PD-L1 blockade leads to marked tumour regression in murine models of small-cell lung cancer (SCLC), according to results presented in a Mini Oral Session at the ESMO Targeted Anticancer Therapies Congress 2022.
SCLC tumours have relatively low immunogenicity. Current treatment recommendations for extensive-stage SCLC, which include the addition of an anti-PD-L1 inhibitor to platinum plus etoposide chemotherapy (Ann Oncol. 2021;32:839–853), have met with only small increases in survival. Characterised by p53 loss, SCLC tumours rely on G2/M cell cycle regulator proteins, including WEE1, for survival and so the incorporation of WEE1 inhibitors into treatment strategies provides an opportunity to enhance tumour response.
Using a panel of SCLC models, inhibition of WEE1 was shown to induce G2/M cell cycle arrest, DNA damage and cytosolic DNA accumulation (Abstract 7MO). It also led to activation of the STING-TBK1-IRF3 pathway, with consequent increases in type I interferons (IFN; IFN-α and IFN-β) and pro-inflammatory cytokines, resulting in an immune response mediated by CD8+ cytotoxic T-cells. In addition, WEE1 inhibition led to activation of the STAT1 pathway and increased type II IFN (IFN-γ) and PD-L1 expression.
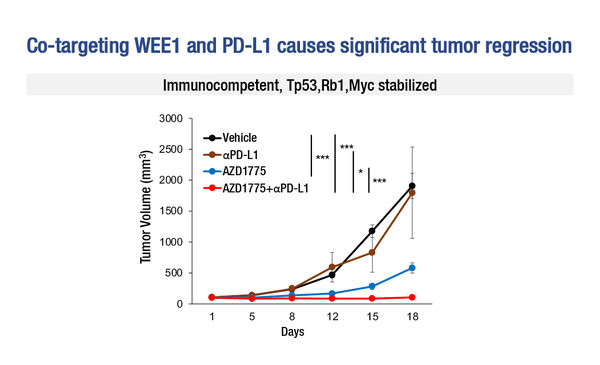
In genetically engineered, immunocompetent murine SCLC models – including a MYC-stabilised aggressive tumour model – the combination of adavosertib with PD-L1 inhibition led to tumour regression that was greater than that seen with either agent alone, along with activation of type I and II IFN pathways and infiltration of cytotoxic T-cells.
“This study provides proof of concept of the synergistic anticancer effect resulting from combining WEE1 inhibition with PD-L1 blockade in SCLC,” says Prof. Laurence Zitvogel from Institut Gustave Roussy, Villejuif, France. “The aim is to bridge a pathway between mitotic catastrophe and the immune system. The results led the researchers to conclude that WEE1 represents a tumour-intrinsic checkpoint inhibitor of immunogenic cell death,” she says. Elaborating further, Zitvogel explains: “In this study, the accumulation of cytosolic DNA that accompanied WEE1-induced cell cycle arrest and apoptosis triggered STING activation and the release of IFN-associated chemokines, which are instrumental in turning ‘cold’ tumours into ‘hot’ tumours.” The study supports a role of immune STING and STAT1 pathways in DNA-damage response (DDR)-mediated antitumour immunity in SCLC. “The new findings are an extension of previous work from the same research group using similar SCLC models, which reported that targeting the DDR proteins PARP and checkpoint kinase 1 (CHK1) significantly increased protein and surface expression of PD-L1, potentiating the antitumour effect of PD-L1 blockade and augmenting cytotoxic T-cell infiltration through the same mechanism of action as WEE1 inhibition (Cancer Discov. 2019;9:646–661),” says Zitvogel.
Abstract discussed:
Sen T. WEE1 inhibition enhances the antitumor immune response to PD-L1 blockade by the concomitant activation of STING and STAT1 pathways in small cell lung cancer. ESMO Targeted Anticancer Therapies Congress 2022, Abstract 7MO
Mini Oral Session, 07.03.2022, h. 17:15 – 18:20, Channel 1