In a post hoc analysis of the DeFi trial, a reduction in symptom burden was reported, with benefits occurring early in treated patients
Patients with desmoid tumour and a stable disease (SD) had significantly improvement of pain, cancer-specific symptom burden, physical functioning, role functioning, and overall quality of life when treated with the oral gamma secretase inhibitor nirogacestat, as presented at the ESMO Sarcoma and Rare Cancers Congress 2024 (Lugano, Switzerland, 14–16 March) (Abstract 57MO).
Nirogacestat is the first treatment for desmoid tumors approved by the U.S. Food and Drug Administration (FDA) based on the benefits showed in terms of progression-free survival (PFS) and objective response rate (ORR) in the international, multicentre, randomised, double-blind controlled DeFi trial where the gamma secretase inhibitor led to a significant 71% reduction in the risk of progression and an increase in objective response rate compared to placebo. Additionally, efficacy results were supported by change from baseline in patient-reported worst pain favoring the nirogacestat arm.
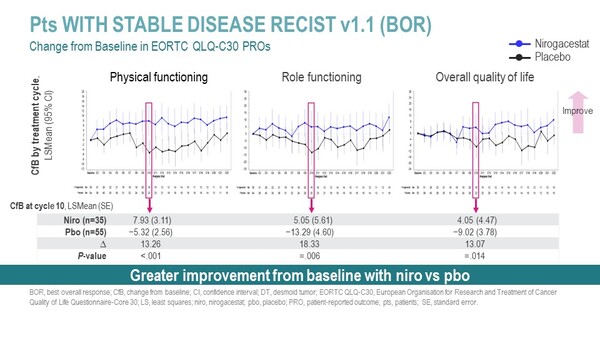
In a post hoc analysis of the DeFi trial, patient-reported outcomes (PROs) of 35 patients with SD as best overall response (BOR) by RECIST v1.1 were collected at baseline and monthly from cycle 2 to 23. Tools used to assess cancer burden were Brief Pain Inventory-Short Form (BPI-SF), Gounder/Desmoid Tumor Research Foundation Desmoid Symptom/Impact Scale (GODDESS®) and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30d (EORTC QLQ-C30), and a change from baseline at cycle 10 was set as a key secondary endpoint.
The analysis findings showed a significant and clinically meaningful improvement in PROs in patients treated with nirogacestat compared to placebo, with responses occurring early in the treatment course and maintained throughout the duration of the study. At cycle 10, a reduction in the average pain intensity was reported in the nirogacestat group compared to the placebo group (–1.48 vs 0.13; P<0.001) and overall quality of life was increased in patients treated with the gamma secretase inhibitor compared to placebo (4.05 vs –9.02: P=0.14).
Desmoid tumours are rare, with an incidence of 3-5 cases per million people worldwide each year. Their clinical behavior is highly variable and, despite of a spontaneous growth arrest and regression rate seen in up to 20% of cases (Clin Cancer Res. 2022 Sep 15;28(18):4092-4104), patients experience a high symptom burden and an impaired quality of life also when the disease is stable or not progressing (Adv Ther. 2023; 40(9): 3697–3722).
Abstract discussed:
Stacchiotti S, et al. Impact of nirogacestat (niro) on patient-reported outcomes (PROs) in adults with desmoid tumor with a best overall response (BOR) of stable disease (SD): Post hoc analysis from the DeFi study . ESMO Sarcoma and Rare Cancers Congress 2024, Abstract 57MO
Mini Oral Session, 15.03.2024, h. 13:00 – 14:00, Hall A