Two studies in sarcoma explore the promise of ADCs and theranostics realised in different tumours.
The heterogeneity of sarcomas and their pathogenesis has challenged the search for more effective treatments. New targets and agents with different mechanisms of action are urgently needed and two studies presented at the ESMO Sarcoma and Rare Cancers Congress 2024 (Lugano, 14–16 March) explore innovative target-specific approaches.
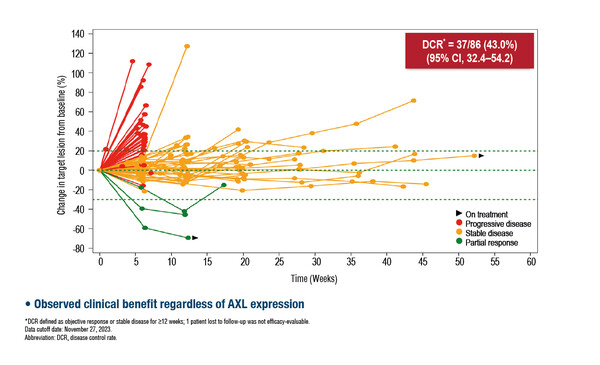
A phase II trial showed promising results with mecbotamab vedotin, an AXL-targeted antibody–drug conjugate (ADC), which is a conditionally active biologic (CAB), meaning it acts only in the acidic tumour microenvironment (Abstract 53O). The disease control rate (DCR) was 43.0% with mecbotamab vedotin monotherapy given every 2 weeks in 86 efficacy-evaluable patients with advanced AXL-expressing refractory sarcoma, many of whom had received three previous systemic therapies. In 11 efficacy-evaluable patients with osteosarcoma, two partial responses were achieved and the 12-week progression-free survival rate was 45.5%. The most common grade ≥3 treatment-emergent adverse events were decreased neutrophil count (10%), abdominal pain (6%) and anaemia (5%).
Prof. Piotr Rutkowski from the Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland, comments: “ADCs have transformed the treatment of other tumour types, such as breast cancer. The finding that AXL is commonly expressed in several of the most common sarcomas means that AXL-targeted ADCs may be beneficial in the treatment of different subtypes, rather than just one particular sarcoma, and provides a biomarker to guide treatment making it a kind of agnostic therapy in several sarcoma subtypes. A DCR of around 40% is promising and, together with seemingly manageable toxicity, these results open the door for further development, perhaps in combination with standard chemotherapy.” Mecbotamab vedotin is being further investigated, particularly among patients with osteosarcoma and undifferentiated pleomorphic sarcoma, in a continuation of the same phase II trial.
The other presentation explored the potential benefits of a theranostics approach in patients with heavily pre-treated soft tissue sarcomas (Abstract 60MO). Patients were screened with gallium-68 prostate-specific membrane antigen (PSMA)-11 PET/contrast-enhanced CT and, if a specific level of uptake was detected, they were treated with lutetium-177 PSMA radioligand therapy. Results from 14 patients indicate high PSMA-tracer accumulation, particularly in those with aggressive sarcomas, with no serious adverse events related to radioligand therapy reported.
“PSMA-targeted theranostics is already becoming a part of treatment for prostate cancer (J Nucl Med. 2022;63:1642–1643) and high expression of PSMA in some sarcomas makes it a promising concept,” says Rutkowski. “The presented results are preliminary, but the fact that there was PSMA accumulation indicates that further investigation is warranted, particularly regarding whether there are certain sarcoma types that are more sensitive than others. In addition, we know that nuclear medicine treatment is generally well tolerated, and the study results appear to confirm this, although access to PET scanners and nuclear medicine facilities may hold theranostics back in under-resourced settings,” concludes Rutkowski.
Abstracts discussed:
Pollack S, et al. Results from a phase 2 part 1 trial of mecbotamab vedotin (BA3011), a CAB-AXL-ADC, in patients with advanced refractory sarcoma. ESMO Sarcoma and Rare Cancers Congress 2024, Abstract 53O
Proffered Paper Session 1, 14.03.2024, h. 10:30 – 12:00, Hall B1
Digklia A, et al. Theranostics in soft tissue sarcoma using a vascular disruption approach: preliminary results of a proof-of-concept pilot study. ESMO Sarcoma and Rare Cancers Congress 2024, Abstract 60MO
Mini Oral Session, 15.03.2024, h. 13:00 – 14:00, Hall A