Different combinations of immunotherapy agent and radiotherapy or chemoradiotherapy were tested in some patient populations in two studies
Two studies presented at the ESMO Immuno-Oncology Congress 2024 (Geneva, 11–13 December) attempted to optimise the use of immunotherapy in different populations of patients with unresectable stage III non-small cell lung cancer (NSCLC).
The phase III CheckMate 73L trial found no benefit of adding immunotherapy to concurrent chemoradiotherapy (cCRT) followed by immunotherapy versus the present standard of care, cCRT then immunotherapy (Abstract 65O). Median progression-free survival (PFS) was not significantly improved with nivolumab plus cCRT followed by nivolumab plus ipilimumab compared with cCRT followed by durvalumab (16.7 months versus 15.6 months, respectively; hazard ratio [HR] 0.95; 96% confidence interval [CI] 0.77–1.19; p=0.6460). PFS was also not improved with nivolumab plus cCRT followed by nivolumab versus cCRT followed by durvalumab (HR 0.84; 95% CI 0.69–1.04). Descriptive overall survival (OS) analysis across the 925 patients also showed no benefit.
As well as the lack of improved efficacy, rates of grade 3–4 treatment-related adverse events (AEs) were higher in the experimental arms (nivolumab plus cCRT followed by nivolumab plus ipilimumab: 57%; nivolumab plus cCRT followed by nivolumab: 54%) compared with the standard-of-care arm (49%). Treatment-related deaths occurred in 3% of patients in both experimental arms and <1% in the standard-of-care arm.
“With higher levels of toxicity and similar efficacy, results from the CheckMate 73L trial add to accumulating evidence that giving immunotherapy with cCRT is not the answer to improving outcomes,” says Prof. Alastair Greystoke from the Northern Centre for Cancer Care, Newcastle University, UK, who continues: “Although the door should now be closed on that particular strategy, stage III lung cancer is a very active research area and a number of ongoing trials are assessing novel immunomodulatory treatments with checkpoint inhibition to boost durability.” He highlights two trials of new combination consolidation therapies that are currently recruiting: PACIFIC-8 studying durvalumab plus domvanalimab (targeting TIGIT) (NCT05211895) and PACIFIC-9 evaluating durvalumab with either oleclumab (targeting CD73) or monalizumab (targeting NKG2A) (NCT05221840).
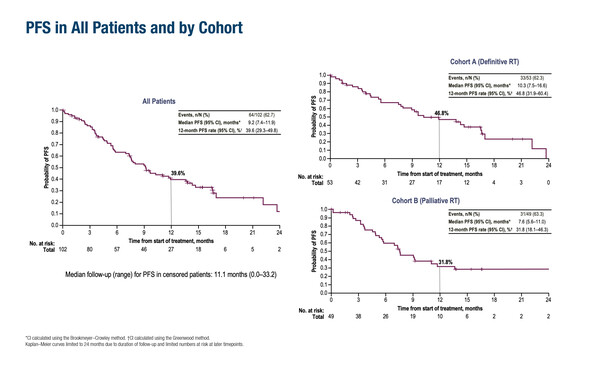
Greystoke also points to the large unmet need of patients with unresectable stage III NSCLC ineligible for cCRT. “The PACIFIC trial, which established the benefit of durvalumab consolidation therapy following cCRT, included only patients with Eastern Cooperative Oncology Group performance status (ECOG PS) 0 and 1 (N Engl J Med. 2017;377:1919–1929), but there are many older patients and those with comorbidities who cannot tolerate cCRT,” he observes. As such, the final analysis of the phase II DUART trial – which suggested some benefit from administering durvalumab after RT in older, frailer patients ineligible for chemotherapy – provides important insights (Abstract 66MO). The primary endpoint of grade 3–4 possibly treatment-related AEs within 6 months of the first durvalumab dose occurred in 9.8% of patients (definitive RT plus durvalumab: 9.4%; palliative RT plus durvalumab: 10.2%). After a median treatment duration of 36 weeks, median PFS was 10.3 months with definitive RT plus durvalumab and 7.6 months with palliative RT plus durvalumab, while median OS was 21.1 months and 16.8 months, respectively. Confirmed objective response rates were 34.0% in the definitive RT cohort and 24.5% in the palliative RT cohort. The trial involved 102 patients with a median age of 79 years. ECOG PS was 1 in 73.3% of patients and 2 in 7.9% of patients.
“The PACIFIC-6 phase II trial had already suggested that durvalumab following sequential CRT was safe in a frailer population (J Thorac Oncol. 2022;17:1415–1427),” notes Greystoke. “Now, with DUART, we have evidence for the efficacy of durvalumab following RT, particularly in the palliative RT cohort who traditionally fare badly. These data provide confidence in the approach and set a benchmark for much-needed randomised phase III studies,” he concludes.
Programme details
Peters S, et al. CheckMate 73L: Phase 3 study comparing nivolumab (N) + concurrent chemoradiotherapy (CCRT) followed by N ± ipilimumab (I) v CCRT followed by durvalumab (D) for previously untreated, locally advanced stage (stg) III NSCLC. ESMO Immuno-Oncology Congress 2024, Abstract 65O
Proffered Paper Session, 11.12.2024, h. 16:15 – 18:00, Room B
Filippi AR, et al. Durvalumab after radiotherapy (RT) in patients (pts) with unresectable stage III NSCLC ineligible for chemotherapy (CT): final analysis of the phase 2 DUART study. ESMO Immuno-Oncology Congress 2024, Abstract 66MO
Mini Oral Session 2, 12.12.2024, h. 14:15 – 15:45, Room B