A lack of benefit coupled with increased toxicity was reported in patients with stage I/II inoperable disease in the KEYNOTE-867 trial
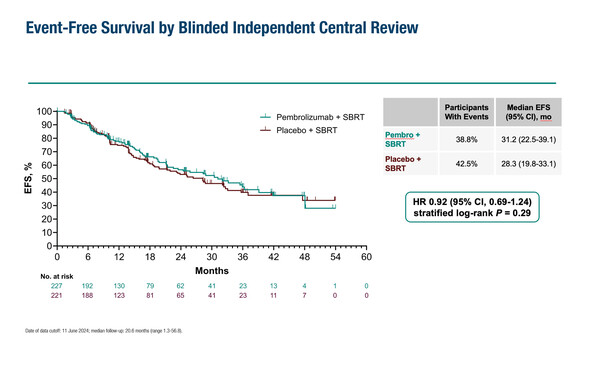
As presented at the ESMO Immuno-Oncology Congress 2024 (Geneva, 11–13 December), median event-free survival was not significantly improved with pembrolizumab versus placebo given concurrently with stereotactic body radiotherapy (31.2 months versus 28.3 months; hazard ratio 0.92; 95% confidence interval 0.69–1.24; p=0.29) (Abstract 117O). The KEYNOTE-867 trial involved 448 patients who were unable to, or refused surgery, or were diagnosed with inoperable stage I/II non-small cell lung cancer (NSCLC).
Grade ≥3 treatment-related adverse events (AEs) occurred in 20.4% of patients in the pembrolizumab arm and 3.7% of patients in the placebo arm, with 5 and 0 treatment-related deaths, respectively. Grade ≥3 immune-mediated AEs and infusion reactions occurred in 9.3% of patients in the pembrolizumab arm, with 3.1% of patients experiencing grade ≥3 treatment-related pneumonitis. Based on the benefit–risk profile, the trial was stopped early.
“After the PACIFIC trial established durvalumab consolidation therapy following chemoradiotherapy as the standard of care in locally advanced NSCLC (N Engl J Med. 2017;377:1919–1929), it was proposed that administering immunotherapy at the same time as radiotherapy may boost the immune response and prevent relapse, which is relatively common in stage I/II NSCLC. However, these results from the KEYNOTE-867 trial indicate that not only does this strategy provide no additional gains, it is also associated with unacceptable toxicity,” comments Prof. Alfredo Addeo from Geneva University Hospital, Switzerland. He points to other recent negative trials – PACIFIC-2 in unresectable stage III NSCLC (Ann Oncol. 2024;9(Suppl_3):LBA1) and NRG-LU005 in limited-stage small cell lung cancer (Int J Radiat Oncol Biol Phys. 2024;120(Suppl):LBA02) – as further evidence that checkpoint inhibition at the same time as (chemo)radiation does not seem to be worth pursuing as a therapeutic approach. “It is not known why concurrent administration with radiation blunts the benefits of immunotherapy but an understanding of the mechanism and how best to schedule immunotherapy with radiotherapy may help us to make progress in treating early-stage NSCLC,” Addeo concludes.
Programme details
Pircher A, et al. Stereotactic body radiotherapy (SBRT) with pembrolizumab (pembro) for unresected stage I/II non-small-cell lung cancer (NSCLC): The randomized, double-blind, phase 3 KEYNOTE-867 study. ESMO Immuno-Oncology Congress 2024, Abstract 117O
Proffered Paper Session, 11.12.2024, h. 16:15 – 18:00, Room B