Different methods – multiparametric biomarker assessment and FDG-PET – show mixed accuracy and their applicability in clinical practice needs to be assessed
At ESMO Immuno-Oncology Congress 2022 (Geneva, 7–9 December), two studies investigated the feasibility and utility of novel biomarkers based on data from patients with non-small-cell lung cancer (NSCLC) receiving immune checkpoint inhibitors alone or in combination with chemotherapy.
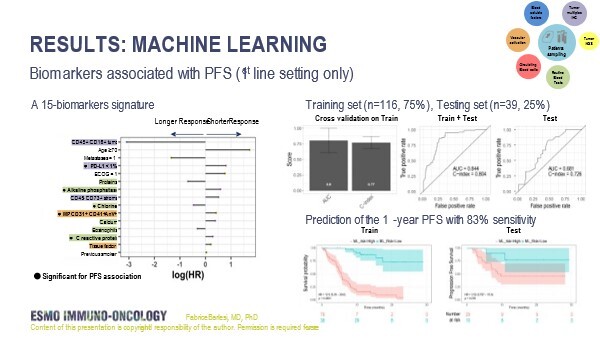
In the first study, the PIONeeR trial, a comprehensive multiparametric and longitudinal biomarker assessment of tumour tissue and blood was used to assess response to first-line pembrolizumab plus platinum-based chemotherapy (Abstract 3MO). In a subgroup analysis of 155 patients with ECOG performance status (PS) 0–1, supervised machine learning on 298 biomarkers revealed a 15-biomarker signature comprising classical parameters (including age, ECOG PS and PD-L1 tumour proportion score) and experimental parameters (including CD45+ CD16+ cell density in tumour tissue, CD45− CD73+ cell density in stromal tissue, tissue factor and vascular microparticles). The signature showed a high predictive performance for progression-free survival (PFS) at 1 year on a training set of data (n=116) (C-index 0.77 and area under the curve [AUC] 0.80), which was confirmed on a testing dataset (n=39) (C-index 0.73 and AUC 0.68).
“These results show that combining biomarkers is likely to improve accuracy since it is highly likely that no single biomarker alone will have sufficient predictive capacity,” explains Prof. Luca Mazzarella from the European Institute of Oncology, Milan, Italy, who cites the use of a time-to-event endpoint and the number of biomarkers investigated as positive aspects of the study design.
“This is probably the largest number of biomarkers achievable with current technologies, providing a comprehensive picture of the tumour microenvironment (including parameters quantifying vascular activation), the tumour and host genetic landscape, and the pharmacokinetic/pharmacodynamic parameters – all factors that may impact on response,” he says. However, given that these are preliminary data, there are issues that need to be addressed. “The small number of patients included in the study relative to the number of biomarkers effectively reduces the statistical power,” he adds. “And while the accuracy rate of around 80% is encouraging, a higher rate would be required for a biomarker combination to be considered for use in routine clinical practice, particularly as multidimensional data significantly increase the cost.” He also points to the small validation test cohort and the lack of external validation, making it impossible to discount the influence of random factors on the findings. Despite these caveats, Mazzarella thinks this large multidimensional study represents a significant advance towards the identification of the most informative biomarkers for use in the clinical setting.
In the other study presented, among 488 patients with advanced NSCLC, 18FFDG PET-determined metabolic tumour volume (MTV) was negatively correlated with median PFS in patients receiving immune checkpoint inhibitors alone (p<0.001) and, more weakly, in patients receiving immune checkpoint inhibitors in combination with chemotherapy (p=0.037), but not in patients receiving chemotherapy alone (p=0.696) (Abstract 4P). Among patients with PD-L1 ≥50%, combined immune checkpoint inhibitor/chemotherapy treatment was associated with significant PFS and overall survival advantages compared with immune checkpoint inhibitors alone in patients with MTV greater than the median (100 cc) but not in patients with MTV less than the median. Transcriptomic analysis on 48 patients showed a negative correlation between MTV and immune signatures and Cybersort-based immune infiltrate quantification (p=0.016).
The study confirms findings from previous studies (J Immunother Cancer. 2020;8:e000645) on the potential utility of MTV as a biomarker. “The results are strengthened by the lack of association with outcome in the chemotherapy cohort, which acts as a control group, and by the good correlation with immune transcriptional analyses. This suggests that the association between MTV and PFS is not simply prognostic, but specifically predictive of response to immune checkpoint inhibitors,” says Mazzarella. He also highlights the immediate clinical applicability of this approach as 18FFDG-PET is a routine analysis in lung cancer, although standardisation of the technique is still required.
He concludes: “In the last few years, we have seen several studies uncovering the potential of high-granularity methods, such as transcriptomics, imaging and dynamic biomarkers. These studies should help us to identify biomarkers that are not only informative but that lead to the development of economically viable and sustainable assays, allowing them to be used for all our patients.”
Abstract discussed:
Barlesi F, et al. Comprehensive biomarkers (BMs) analysis to predict efficacy of PD1/L1 immune checkpoint inhibitors (ICIs) in combination with chemotherapy: a subgroup analysis of the Precision Immuno-Oncology for advanced Non-Small CEll Lung CancER (PIONeeR) trial. ESMO Immuno-Oncology Congress 2022, Abstract 3MO
Mini Oral Session 2 08.12.22, h. 14:30 – 15:35, Room B. Also watch the session on the Congress virtual platform
Dall'Olio FG, et al. FDG PET derived Metabolic Tumor Volume (MTV) and its transcriptomic correlates as biomarker to predict efficacy of immune checkpoint inhibitors (ICB) alone or in combination with chemotherapy in advanced NSCLC: A multicentric study. ESMO Immuno-Oncology Congress 2022, Abstract 4P
Poster Display 08.12.22, h. 12:30 – 13:15, Foyer mezzanine. Also watch the session on the Congress virtual platform