Phase II study indicates that SHR-1701 alone or together with chemotherapy may be a valuable induction strategy in stage III unresectable NSCLC
As reported at ESMO Immuno-Oncology Congress 2022 (Geneva, 7–9 December), induction treatment with the novel anti-PD-L1/TGFβ receptor II (RII) fusion protein, SHR-1701, with or without chemotherapy (paclitaxel plus carboplatin), induced promising antitumour activity and enabled surgery in 25.2% (27/107) of patients with stage III unresectable non-small-cell lung cancer (NSCLC) who were assessed by a multidisciplinary team for definitive surgery or chemoradiotherapy (LBA5). This is one of the first studies to explore chemoimmunotherapy, with a novel agent, as induction therapy, followed by conversion surgery or definitive chemoradiotherapy and SHR-1701 consolidation, in this setting.
For induction, patients with PD-L1 tumour proportion score (TPS) <50% received combination SHR-1701 plus chemotherapy (n=88), while those with PD-L1 TPS ≥50% were randomised (1:1) to receive SHR-1701 plus chemotherapy (n=9) or SHR-1701 monotherapy (n=10). All patients received SHR-1701 as consolidation therapy after definitive surgery or chemoradiotherapy.
The objective response rate (ORR) post induction was 56.1% in all patients, 57.7% in patients receiving combination therapy and 40.0% in patients receiving SHR-1701 monotherapy. The corresponding best ORRs were 70.1% overall, 71.1% with combination therapy and 60.0% with SHR-1701 monotherapy. Grade ≥3 treatment-related adverse events (TRAEs) were reported in 77 (72.0%) of patients. The most common TRAEs occurring in ≥50% of patients were haematotoxicities. No new safety signals were identified.
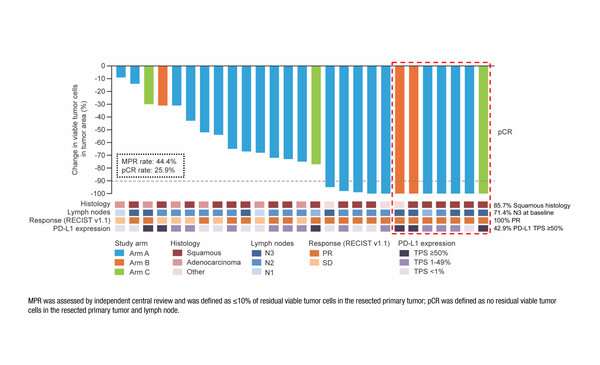
Favourable activity was observed in the limited subgroup of resected patients: 12 (44.4%) had a major pathological response, 7 (25.9%) had a pathological complete response and 14 (51.9%) had nodal downstaging. The median event-free survival was not reached in resected patients.
“Although preliminary, these results are very encouraging,” says Dr Sara Pilotto from the University of Verona Hospital Trust, Italy. “Improving the outcomes of patients with unresectable stage III NSCLC remains a major challenge and, based on the positive results from the CheckMate 816 trial in resectable NSCLC published earlier this year (N Engl J Med. 2022;386:1973–1985), there is a strong rationale for exploring the potential of chemoimmunotherapy in unresectable disease,” she explains. “The fact that nearly a quarter of patients achieved surgery conversion with SHR-1701, mainly in combination with chemotherapy, is particularly noteworthy, but additional data are needed,” says Pilotto.
Preclinical evidence supports the simultaneous targeting of TGFβRII and PD-L1 with a bifunctional molecule, which provides more potent antitumour activity than anti-PD-L1 or anti-TGFβRII treatment administered as single agents or in combination (Nat Commun. 2018;9:741). “However,” cautions Pilotto, “experience with bifunctional proteins shows us that promise in early-phase trials is not always realised in later clinical development (Mol Oncol. 2022;16:2117–2134). We need additional, long-term efficacy data with SHR-1701 – in NSCLC and other cancer types – and, in particular, we need to see more safety data, given that 72% of patients in this phase II trial experienced at least grade 3 TRAEs, commonly haematotoxicity.”
Abstract discussed:
Wu Y.-L, et al. A phase 2 study of neoadjuvant SHR-1701 with or without chemotherapy (chemo) followed by surgery or radiotherapy (RT) in stage III unresectable NSCLC (uNSCLC). ESMO Immuno-Oncology Congress 2022, LBA5
Proffered Paper Session 07.12.22, h. 14:05 –15:40, Room B. Also watch the session on the Congress virtual platform.