Effective biomarkers and histology-focused enrichment trials are now needed to further improve patient outcomes
In the peri-operative treatment of stage III localised soft tissue sarcoma (STS), the addition of immunotherapy combinations to radiotherapy (RT) may improve pathological response through a synergistic mechanism by amplifying T-cell priming and cancer cell killing following increased release of tumour immune antigens induced by pre-operative RT (Onco Targets Ther. 2023;16:385–397).
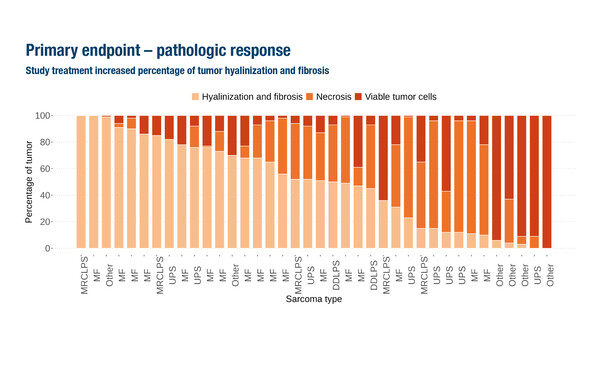
A demonstration comes from the phase II EFTISARC-NEO study, which met its primary endpoint of pathological response with an increase in percentage tumour hyalinisation and fibrosis in patients with stage III STS treated with the LAG-3 agonist, eftilagimod alpha, and pembrolizumab with concurrent RT, followed by surgery (Abstract 2686O, key results in the box below). As presented at the ESMO Congress 2025 (Berlin, 17–21 October), median tumour hyalinisation and fibrosis was 51.5% (interquartile range [IQR] 15–76; p<0.001) in 38 evaluable patients with STS of the extremities or trunk. The tumour hyalinisation and fibrosis rate was substantially greater than that reported historically with pre-operative RT alone (~15%) and also greater than the 35% predicted by study statistical assumptions, so the primary endpoint was met. Median residual viable tumour was 12.5% (IQR 4.5–30).
Commenting on the data, Dr Sandra P. D’Angelo from the Memorial Sloan Kettering Cancer Center, New York, USA notes that while 47% of patients had <10% viable tumour cells, the objective response rate (ORR; all partial responses) per RECIST was only 13.2%. In addition, treatment-related adverse events (TRAEs) occurred in 92.5% of patients, with grade ≥3 TRAEs in 20.0%. However, the rate of post-operative complications was consistent with rates observed following pre-operative RT alone.
“The findings are comparable to a previous study (SARC032) that investigated the addition of pembrolizumab to pre-operative RT followed by surgery in patients with stage III STS (Lancet. 2024;404:2053–2064),” she says. “One important caveat is that in both the SARC032 and EFTISARC-NEO studies, at least two different subtypes were enrolled, as is common in trials with rare cancers. This results in small numbers of patients per histology subtype, specifically liposarcoma in SARC032 as well as myxoid liposarcoma in EFTISARC-NEO. Histology-focused enrichment trials with sufficient patients of a given subtype are required to further evaluate the efficacy of specific therapies,” she says.
Combining peri-operative immunotherapy with RT may be a compelling alternative to RT alone for selected sarcoma histologies, but it is currently unclear whether the addition of eftilagimod alpha is better than pembrolizumab alone plus RT, or whether the combination is more efficacious than neoadjuvant chemotherapy. However, D’Angelo emphasises that given the limitations of chemotherapy, including the associated toxicities that prevent older patients (>65 years) from receiving treatment, there will be a place for novel immunotherapy combinations for patients with specific sarcoma histologies such as undifferentiated pleomorphic sarcoma/myxofibrosarcoma.
On this rationale, the phase III RAR-IMMUNE study evaluated nivolumab plus ipilimumab compared with pazopanib in 116 patients with 16 different types of rare (defined as <5% of all sarcomas), advanced sarcomas. In the final analysis presented at the ESMO Congress, the study did not reach its primary endpoint as no significant difference was observed in progression-free survival (PFS) between arms: median PFS was 3.8 months with nivolumab plus ipilimumab versus 4.0 months with pazopanib (p=0.88) (LBA98, key results in the box below). Median OS and ORR were 18.6 months and 15.3% with nivolumab plus ipilimumab and 18.5 months and 5.3% with pazopanib. D’Angelo notes that response rates were not presented by histology, making it difficult to draw comparisons with other studies, although efficacy is similar to previous reports with immunotherapy in patients with angiosarcoma (25% ORR with dual immune checkpoint inhibitor therapy; Curr Treat Options Oncol. 2025;26:242–250) and epithelioid sarcoma (~15% ORR with anti-PD-(L)1; J Clin Oncol. 2025;43(Suppl):e23558). Similarly, pazopanib has been associated with an ORR of 6%, median PFS of 4.6 months and OS of 12.5 months in patients with metastatic non-adipocytic soft-tissue sarcoma after failure of standard chemotherapy (Lancet 2012;379:1879–1886).
“A better understanding of biomarkers of response will provide additional insights,” comments D’Angelo. “Many studies have explored immune checkpoint blockade and VEGF inhibition in sarcoma and produced similar results. Therefore, effective biomarkers and specific selection criteria, in addition to more effective novel therapy combinations, will be needed to further improve outcomes for patients with different sarcoma histologies.”
The presence of tertiary lymphoid structures (TLS) is an emerging predictive biomarker of response to PD-1 inhibition in STS (Nat Med. 2022;28:1199–1206) that was further evaluated in the phase II randomised CONgRAtS study of 63 patients with TLS-positive STS treated with dual PD-1 plus LAG-3 blockade (nivolumab plus relatlimab) or anti-PD-1 alone (Abstract 2687MO, key results in the box below). The study met a pre-defined primary efficacy threshold in both arms as the 6-month progression-free rate (PFR), the primary endpoint, was 26.7% with nivolumab plus relatlimab and 39.4% with nivolumab alone. Median PFS was 3.7 months and 5.2 months, and median OS was 12.6 months and 29.0 months in nivolumab plus relatlimab and nivolumab arms, respectively. While the addition of relatlimab did not improve efficacy over anti-PD-1 monotherapy, the results of both arms exceeded historical benchmarks, suggesting that TLS may have clinical value in guiding immunotherapy.
“As a biomarker, TLS has proven to be quite promising, yet it is present in ~20% of patients with STS, while testing can be challenging and is not streamlined across centres, and overall response rate to immunotherapy is about 30% in TLS-positive patients (Nat Med. 2022;28:1199–1206),” concludes D’Angelo. “However, it is one of the first biomarkers in sarcoma that has really begun to drive the field forward towards patient selection, and while it needs refinement to optimise responses, it provides hope for the future treatment of this disease.”
At a glance:
Kozak K, et al. EFTISARC-NEO: A phase II study of neoadjuvant eftilagimod alpha, pembrolizumab and radiotherapy in patients with resectable soft tissue sarcoma. ESMO Congress 2025 - Abstract 2686O
- N=40 (38 evaluable pts)
- Median percent hyalinisation/fibrosis: 51.5%
- Median residual viable tumour: 12.5%
- ORR: 13.2%; DCR: 92.1%
- Grade ≥3 TRAEs: 20%
- AEs related to surgery: 87.5%
- DFS and OS data are currently immature
Brahmi M, et al. Final results of the RAR-IMMUNE study: a randomized, comparative, prospective, multicenter phase III trial of the efficacy of nivolumab + ipilimumab (N+I) versus pazopanib (P) in patients (pt) with advanced sarcoma (S) of rare subtype. ESMO Congress 2025 -LBA98
- N=116 (N + I n=59; P: n=57)
- Most common histologies: chordoma (21%), solitary fibrous tumour (13%), angiosarcoma (10%), epithelioid sarcomas (7%), desmoplastic small round-cell tumour (7%) and epithelioid haemangioendothelioma (7%)
- N + I vs P:
- mPFS: 3.8 mo vs 4.0 mo
- mOS: 18.6 mo vs 18.5 mo
- ORR: 15.3% vs 5.3%
- Grade ≥3 TRAEs: 23.7% vs 47.4%
Peyraud F, et al. CONgRAtS: a randomized phase II study of nivolumab±relatlimab in patients with TLS-positive soft-tissue sarcomas. ESMO Congress 2025 - Abstract 2687MO
- N=63 TLS+ (nivo + relatlimab n=30; nivo n=33)
- Nivo + relatlimab vs nivo:
- 6-mo PFR: 26.7% vs 39.4%
- mPFS: 3.7 mo vs 5.2 mo
- mOS: 12.6 mo vs 29.0 mo
- Grade ≥3 TRAEs (N=68): 14.7% vs 14.7%