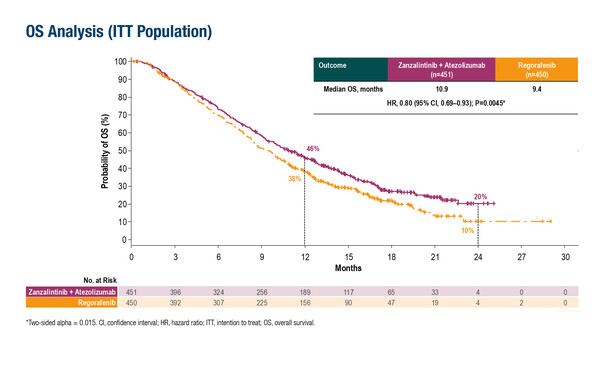
Primary phase III overall survival analysis suggests that the combination therapy may be a potential new option for previously treated patients with or without liver metastases
Patients with refractory metastatic colorectal cancer (mCRC) and microsatellite stable (MSS) disease have limited treatment options as they do not respond to immunotherapy, which is generally associated with durable responses in this setting (JAMA Netw Open. 2025;8:e251186; ESMO Open. 2024;9:103483). Zanzalintinib plus atezolizumab emerges as a potential novel treatment strategy according to the first findings of the phase III STELLAR-303 study, presented at the ESMO Congress 2025 (Berlin, 17–21 October) (LBA30).
In preclinical research, the multitargeted tyrosine kinase inhibitor (TKI) zanzalintinib increased pro-inflammatory immune cells and decreased immunosuppressive cells (Mol Cancer Ther. 2023;22:179–191), suggesting immunomodulatory properties that may enhance response to immune checkpoint inhibitors (ICIs) in MSS disease (Future Oncol. 2024;20:1733–1743). Also, promising antitumour activity and manageable toxicity have been demonstrated with the agent in combination with atezolizumab in patients with refractory MSS mCRC (J Clin Oncol. 2025;43(Suppl):127).
In STELLAR-303, 901 patients were randomised to receive either zanzalintinib plus atezolizumab, or regorafenib – the current standard of care for refractory MSS mCRC. Data presented showed that the TKI–anti-PD-L1 combination significantly improved median overall survival (OS) when compared with regorafenib in patients with previously treated mCRC (10.9 months versus 9.4 months, respectively; hazard ratio [HR] 0.80; 95% confidence interval [CI] 0.69–0.93; p=0.0045).
OS benefits were consistent across pre-specified subgroups, including geographic region, RAS status, prior anti-VEGF therapy and presence or absence of liver metastases. Dr Elena Elez from the Vall d'Hebron University Hospital and Vall d'Hebron Institute of Oncology, Barcelona, Spain notes that, “This is a prospective phase III study to investigate treatment efficacy in refractory MSS mCRC with dual primary endpoints of OS in the intent-to-treat population and in patients without active liver metastases. It was previously demonstrated that patients with treatment-resistant MSS may have more clinical benefit from ICI-based combinations in the absence of liver metastases (JAMA Netw Open. 2021;4:e2118416).” In this study, zanzalintinib plus atezolizumab showed activity in patients irrespective of presenting with liver disease.
Median progression-free survival was also improved with the combination versus regorafenib (3.7 months versus 2.0 months, respectively; HR 0.68; 95% CI 0.59–0.79). According to Elez, however, the magnitude of clinical benefit is limited. “This is not uncommon in the setting of refractory disease, particularly with immunotherapy in patients with MSS disease and liver metastasis. The next step will be to understand which patients derive most benefit: further biomarker research is needed. Outcomes of the final analysis of OS particularly in patients without active liver metastasis will also help to clarify the role of zanzalintinib plus atezolizumab in the current treatment landscape considering the safety profile, which is fundamental in this heavily pre-treated population,” she concludes.
Programme details
Saeed A, et al. Zanzalintinib plus atezolizumab (ZANZA + ATEZO) vs regorafenib (REGO) in patients (pts) with previously treated metastatic colorectal cancer (mCRC): Primary overall survival (OS) analysis from the randomized, open-label, phase 3 STELLAR-303 study. ESMO Congress 2025 - LBA30