Potentially practice-changing data were presented for atezolizumab plus bevacizumab, and camrelizumab plus rivoceranib in hepatocellular carcinoma
While transarterial chemoembolisation (TACE) is standard of care for intermediate-stage hepatocellular carcinoma (HCC), some patients continue to progress following the procedure, with deterioration in liver function. Given that repeated procedures can be associated with an increased risk of liver damage (United European Gastroenterol J. 2019;7:850–858), and as many patients are unable to receive TACE due to major contraindications (J Clin Med. 2025;14:314), preliminary data with a combination of atezolizumab and bevacizumab reported at the ESMO Congress 2025 (Berlin, 17–21 October) are particularly encouraging (LBA51, key results in the box below).
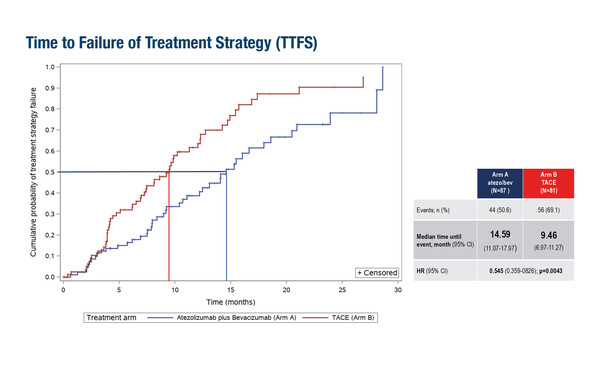
In a first interim analysis of ABC-HCC, a phase IIIb trial of 168 patients with intermediate-stage HCC not amenable to curative surgery/ablation, the median time to failure of treatment strategy, the primary endpoint, was 14.6 months in the combination arm compared with 9.5 months with TACE (hazard ratio [HR] 0.55; 95% confidence interval [CI] 0.36–0.83; p=0.0043).
Atezolizumab plus bevacizumab previously demonstrated improved overall survival compared with sorafenib (standard of care at the time) as a first-line treatment for locally advanced or metastatic and/or unresectable HCC in a global phase III trial (N Engl J Med. 2020;382:1894–1905; J Hepatol. 2022;76:862–873), and was subsequently approved for this indication, so there is good rationale for investigation of this systemic treatment combination in earlier stages of disease.
“Many patients are unable to receive TACE, with major contraindications including extensive bilobar tumour load, decompensated liver disease and severe comorbidities,” explains Dr Lorenza Rimassa from the Humanitas University and IRCCS Humanitas Research Hospital, Milan, Italy. “This finding could be practice changing if the final analysis is positive.”
A second combination therapy reported positive findings for earlier-stage HCC. In an interim analysis of the randomised phase III CARES-009 study, the anti-PD1 antibody camrelizumab plus rivoceranib, a selective VEGFR2 inhibitor, showed a statistically significant and clinically meaningful benefit compared with surgery alone as peri-operative treatment for 294 patients with resectable HCC at intermediate or high risk of recurrence (Abstract 1470O, key results in the box below).
The combination is not approved for use in Europe but was previously demonstrated to have activity in the advanced setting (Lancet. 2023;402:1133–1146). In the CARES-009 study, peri-operative treatment with camrelizumab plus rivoceranib significantly increased investigator-assessed event-free survival (EFS), the primary endpoint, compared with surgery (42.1 months versus 19.4 months; HR 0.59; 95% CI 0.41–0.85; p=0.004). A higher major pathological response rate (35.1% versus 7.5%, respectively; p<0.001) and prolonged median disease-free survival (40.8 months versus 19.4 months; HR 0.59) were also reported with the peri-operative strategy versus surgery alone. The safety profile of the combination treatment was manageable; grade ≥3 treatment-related adverse events (TRAEs) were reported in 38% of the peri-operative arm. “Unfortunately, the data cannot be generalised to other patients with intermediate/high-risk HCC because they are specific to a Chinese population. We know that HCC may have different aetiologies according to patient geographic location, and in China, 80% of patients with this malignancy have underlying hepatitis B virus (HBV) infection (Liver Int. 2016;36:1582–1584),” notes Rimassa. Should the final analysis be positive, the data may impact peri-operative treatment in the Asian country, but further investigation would be required in an expanded cohort of Western patients to influence clinical practice elsewhere.
Promising data were also presented at the ESMO Congress with a novel neoadjuvant regimen in patients with resectable intrahepatic cholangiocarcinoma (iCCA) at high risk of recurrence, although they will unlikely have a practice-changing impact worldwide. The ‘GOLP’ regimen (gemcitabine and oxaliplatin with lenvatinib and the anti-PD-1, toripalimab) demonstrated longer median EFS, the primary endpoint, than surgical resection alone in an interim analysis of a phase II–III trial of 178 patients (18.0 months versus 8.7 months) (LBA11, key results in the box below). Safety was manageable, with grade ≥3 TRAEs reported in 26% of patients.
Rimassa notes that despite the encouraging findings, “the regimen has not previously demonstrated activity in advanced iCCA worldwide and the activity of lenvatinib has not been demonstrated in this setting. A phase II study with tislelizumab in place of toripalimab recently reported promising efficacy and manageable safety with the regimen in unresectable, locally advanced biliary tract cancer (Lancet Oncol. 2025:S1470-2045(25)00376-6), but the data from this study cannot be applied to Western populations.”
The phase II–III study remains important as it demonstrates activity in a neoadjuvant iCCA setting with a novel systemic regimen. “These data support the concept of systemic neoadjuvant chemotherapy plus immunotherapy in resectable iCCA where there is a significant unmet need,” she concludes. Studies are now ongoing in Western countries to evaluate other regimens in the peri-operative or neoadjuvant settings (Oncol Transl Med. 2025;11:1–4).
At a glance:
Galle PR, et al. IKF-035/ABC-HCC: a phase IIIB, randomized, multicenter, open-label trial of atezolizumab plus bevacizumab versus transarterial chemoembolization (TACE) in intermediate-stage hepatocellular carcinoma. ESMO Congress 2025 - LBA51
- N=168 in first interim analysis (atezo + bev n=87; TACE: n=81)
- Atezo + bev vs TACE:
- mTTFS: 14.6 mo vs 9.5 mo (HR 0.55; 95% CI 0.36–0.83); p=0.0043
- Toxiticty-associated treatment discontinuation (without PD): 8% vs 0%
Zhou J, et al. Perioperative camrelizumab plus rivoceranib in resectable hepatocellular carcinoma (CARES-009): A randomized, multicenter, phase 3 trial. ESMO Congress 2025 - Abstract 1470O
- N=294 (cam + rivo n=148; surgery n=146)
- Median follow-up: 21.3 mo (interim analysis)
- Cam + rivo vs surgery:
- mEFS: 42.1 mo vs 19.4 mo (HR 0.59; p=0.004)
- MPR: 35.1% vs 7.5% (p<0.001)
- DFS: 40.8 mo vs 19.4 mo (HR 0.59)
Shi G, et al. Neoadjuvant toripalimab plus lenvatinib and GEMOX in resectable, high-risk intrahepatic cholangiocarcinoma: A randomized, multicenter, open-label phase III clinical trial. ESMO Congress 2025 - LBA11
- N=178 (neo n=88; surgery: n=90)
- Median follow-up: 16.9 mo (interim analysis)
- Neo vs surgery:
- mEFS: 18.0 mo vs 8.7 mo
- mOS: NE vs 31.4 mo
- pCR: 4.5% vs 0%