Two studies fail to meet their primary endpoints, reinforcing the need to improve the sensitivity of ctDNA testing before it enters the clinic
The tantalising prospect of being able to use the presence/absence of circulating tumour DNA (ctDNA) as a decision-making aid has received much attention in recent years; however, study results presented at the ESMO Congress 2025 (Berlin, 17–21 October) indicate that ctDNA testing does not yet match standard practices.
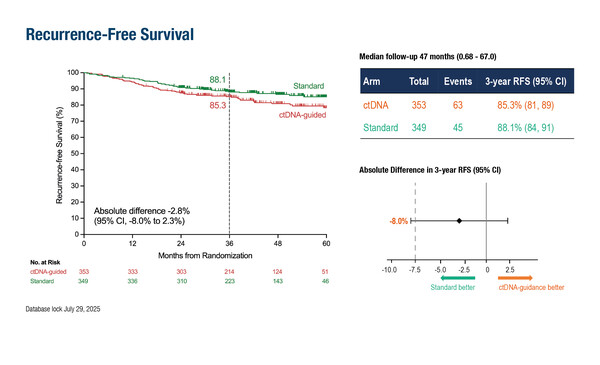
In an analysis of the DYNAMIC-III trial, presented at today’s Presidential Symposium, non-inferiority was not confirmed between ctDNA-guided treatment and standard adjuvant chemotherapy in 702 patients with stage III colon cancer who had undergone resection and were found to be ctDNA-negative (LBA9, key results in the box below). After median follow-up of 47 months, 3-year recurrence-free survival (RFS), the primary endpoint, was 85.3% in the ctDNA-guided arm versus 88.1% in the standard arm (absolute difference –2.8%; 95% confidence interval –8.0–2.3), thus crossing the lower bound of –7.5%.
Commenting on the findings, Prof. Dominik Modest from the Charité University of Medicine, Berlin, Germany, highlights the need for further investigations into the reasons behind these disappointing results. “Gaining more insights into test performance and the factors contributing to the numerically lower RFS rate in the ctDNA arm may help ensure that future treatment decisions maintain appropriate chemotherapy intensity and duration,” he suggests.
In the DYNAMIC-III trial, clinicians nominated the selected adjuvant chemotherapy regimen prior to randomisation to ctDNA-informed or standard management. For ctDNA-informed management, a ctDNA-negative result at 5–6 weeks after surgery with the SaferSeqS targeted CRC panel test prompted a de-escalated adjuvant strategy (from 6 months of single-agent fluoropyrimidine [FP] to no chemotherapy or 3 months of FP; from 3 months of oxaliplatin plus FP to 3–6 months of FP; or from 6 months of oxaliplatin plus FP to 3 months of oxaliplatin plus FP or 6 months of FP). Treatment de-escalation was found to significantly reduce oxaliplatin-based chemotherapy use (34.8% versus 88.6%; p<0.001), in addition to lowering grade ≥3 treatment-related adverse events of special interest (6.2% versus 10.6%; p=0.037).
Final results were presented in Berlin from the phase II PEGASUS trial (Abstract 723O, key results in the box below), which also failed to meet its primary endpoint, due in part to reduced power. In total, 12 relapses (indicating 12% false-negative cases) occurred in 100 ctDNA-negative patients with high-risk stage II or stage III colon cancer at 2 years. Less than 14 relapses in 134 ctDNA-negative patients was set as the statistical cut-off for the primary endpoint, but the trial only included 100 ctDNA-negative patients. Over the population as a whole, the presence versus absence of ctDNA was associated with worse disease-free survival (3 years: 58.4% versus 82.8%, respectively; hazard ratio [HR] 2.70; p=0.0036) and overall survival (79.7% versus 93.8%; HR 3.11; p=0.0383) at a median follow-up of 42 months.
In PEGASUS, a liquid biopsy was performed using the Guardant Reveal assay 2–4 weeks after surgery. ctDNA-positive patients received 3 months of CAPOX, while ctDNA-negative patients received 6 months of CAPE. The liquid biopsy was repeated after one cycle of CAPE and treatment escalated to CAPOX if the test was positive. At the end of adjuvant treatment, the liquid biopsy was repeated, and chemotherapy was adjusted or standard follow-up initiated, as appropriate.
“Across all trials investigating ctDNA tests for this purpose, it is important to clearly report their sensitivity and specificity and assess whether they are sufficiently high to validate each individual test for clinical use,” concludes Modest. “If ctDNA tests cannot be made more robust and sensitive, non-inferiority cannot be achieved.”
At a glance:
Tie J, et al. ctDNA-guided adjuvant chemotherapy de-escalation in stage iii colon cancer: primary analysis of the ctDNA-negative cohort from the randomized AGITG DYNAMIC-III trial (Intergroup Study of AGITG and CCTG). ESMO Congress 2025, LBA9
- 702 ctDNA-negative pts (n=353 ctDNA-guided treatment; n=349 standard management)
- Median follow-up: 47 mo
- ctDNA vs standard management:
- Oxaliplatin-based chemotherapy use: 34.8% vs 88.6%; p<0.001
- Grade ≥3 TRAESI: 6.2% vs 10.6%; p=0.037
- 3-year RFS: 85.3% vs 88.1%
- Results in ctDNA-positive patients have been presented previously (J Clin Oncol.2025;43(16_suppl):3503)
Marsoni S, et al. Post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients: final results of the PEGASUS trial. ESMO Congress 2025. Abstract 723O
- N=135 (n=100 ctDNA–; n=35 ctDNA+)
- Median follow-up: 42 mo
- Relapse rate in ctDNA–: 12% (false-negative cases)
- ctDNA– vs ctDNA+:
- DFS HR 2.70; p=0.0036; 36-mo: 82.8% vs 58.4%
- OS HR 3.11; p=0.0383; 42-mo OS rate: 93.8% vs 79.7%