Despite the presentation of encouraging study findings with novel immunotherapy-based therapies, long-term outcomes are needed to better understand the magnitude of clinical benefit and many questions remain around off-target toxicity and the identification of patients most likely to respond to treatment
Early efficacy and safety results with two novel immunotherapy-based strategies for refractory and treatment-naïve, advanced neuroendocrine carcinoma (NEC) were discussed in a Mini Oral Session at the ESMO Congress 2025 (Berlin, 17–21 October), suggesting that the addition of immunotherapy to chemotherapy improves efficacy compared with chemotherapy alone at the cost of higher toxicity.
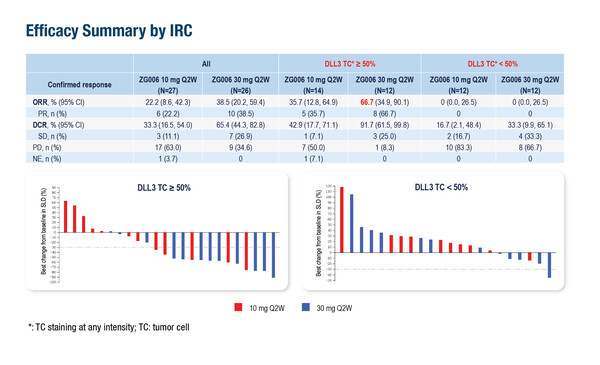
In a phase II dose-expansion study, ZG006, a trispecific T-cell engager targeting two delta-like ligand 3 (DLL3) epitopes on tumour cells and CD3 on T cells, was associated with an objective response rate (ORR) – the primary endpoint – of up to 38.5% in 53 evaluable patients with refractory NEC (60% of whom had received prior anti-PD-[L]1 treatment) (Abstract 1707MO).
“This biparatopic DLL3-targeted trispecific T-cell engager, that binds two DLL3 epitopes plus CD3, can potentially improve T-cell synapse formation on tumours with low or heterogeneous DLL3 density and reduce single-epitope escape, potentially translating into stronger activity at a given CD3 drive. This is indeed an interesting novel and promising approach to target DLL3-expressing tumours such as NECs,” comments Dr Rocio Garcia-Carbonero from the Hospital Universitario 12 de Octubre, Madrid, Spain. “The responses in this refractory patient population are high compared with those typically observed following chemotherapy (~10%), and are seen across a broad spectrum of primary tumour sites, although it is unclear how this relates to DLL3 tumour expression and the quality of responses observed in terms of durability or other outcomes such as progression-free survival (PFS), which were not reported.” Data presented in Berlin were preliminary and still not mature.
A second presentation reported on a novel anti-PD-1/TIGIT bispecific antibody, ZG005, that was given in combination with etoposide and cisplatin as first-line therapy to 62 patients with advanced NEC in a phase I/II study (Abstract 1708MO). In the phase II randomised part of the study, the combination was associated with a higher ORR than chemotherapy alone: 42.9% and 65.0% at the two doses of ZG005 studied, compared with 33.3% with chemotherapy in patients with ≥2 post-baseline tumour assessments (n=7 for 10 mg/kg ZG005, n=20 for 20 mg/kg ZG005 and n=9 for chemotherapy alone). Median duration of response and PFS had not been reached.
“Taking the results from these two studies together, it is clear that these novel immunotherapy strategies, alone or combined with chemotherapy, improve efficacy compared with chemotherapy alone, both in chemotherapy-naive and -refractory patients with advanced NECs. However, toxicity is not negligible, and further information on long-term clinical benefit and toxicity is needed to appropriately assess the risk-benefit balance of these strategies in clinical practice,” adds Garcia-Carbonero.
Grade ≥3 treatment-related adverse events (TRAEs) were experienced by 47% of patients treated with the trispecific T-cell engager – including two cases of grade ≥3 cytokine release syndrome – and serious TRAEs were reported by 23% of patients. Grade ≥3 TRAEs were experienced by 53% of patients and serious TRAEs by 16% of patients who received the anti-PD-1/TIGIT bispecific antibody. Treatment interruption due to TRAEs was required by 14 patients receiving the trispecific T-cell engager, while permanent treatment discontinuation was reported for one patient each with the trispecific T-cell engager and the anti-PD-1/TIGIT bispecific antibody.
Although these results are encouraging and point to addressing unmet needs by enhancing the immune response to NECs, Garcia-Carbonero notes that there are several challenges surrounding the use of novel T-cell targeting agents, not least of which is gaining a better understanding of the associated off-target toxicities. She also stresses the need for a clearer insight of the impact of microsatellite instability and PD-L1 status on efficacy and the identification of novel validated biomarkers to help distinguish patients most likely to respond to PD-1/TIGIT blockade, as currently, patient selection for treatment is limited.
Programme details:
Zhao C, et al. A phase 2 dose expansion study of ZG006, a trispecific T cell engager targeting DLL3/DLL3/CD3, as monotherapy in patients with refractory neuroendocrine carcinoma. ESMO Congress 2025 - Abstract 1707MO
Ye S, et al. ZG005 in combination with etoposide and cisplatin for the first-line treatment of advanced neuroendocrine carcinoma. ESMO Congress 2025 - Abstract 1708MO