Study findings support routine RET-mutation testing for all patients with advanced MTC, but further work is needed to identify predictive markers
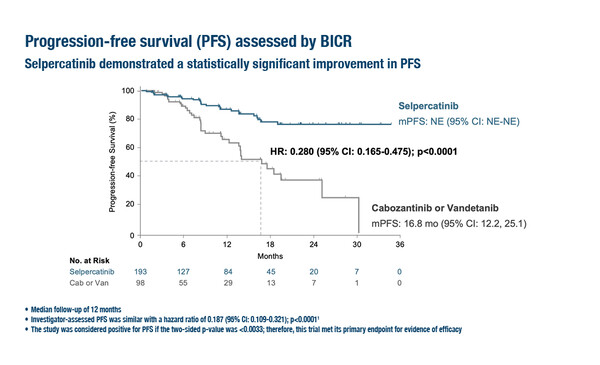
Encouraging data for the treatment of neuroendocrine tumours (NETs) were presented at the ESMO Congress 2023 (Madrid, 20–24 October). In a Presidential Symposium, it was revealed that the phase III LIBRETTO-531 trial met its interim analysis criteria for efficacy, with significantly improved outcomes with first-line selpercatinib, a highly selective and potent RET inhibitor, versus a multikinase inhibitor (MKI) in patients with RET-mutated medullary thyroid carcinoma (MTC) (LBA3). At a median follow-up of 12 months, median progression-free survival (PFS) was not estimable with selpercatinib versus 16.8 months with physician’s choice of MKI (cabozantinib or vandetanib) (hazard ratio [HR] 0.280; 95% confidence interval [CI] 0.165–0.475; p<0.0001) in 291 patients with kinase inhibitor-naïve progressive disease. The overall response rate was 69.4% with selpercatinib versus 38.8% with MKI. Overall survival (OS) at a median follow-up of 15 months was also better with selpercatinib (HR 0.374; 95% CI 0.147–0.949; p=0.0312). Dose reductions (38.9% versus 79.2% with cabozantinib and 72.0% with vandetanib) and permanent treatment discontinuations due to adverse events (4.7% versus 26.8%) were less common with selpercatinib than with MKIs.
“These results provide definitive confirmation that selpercatinib is superior to MKIs from both statistical and clinically relevant perspectives. Tolerability is also better with selpercatinib as shown by fewer dose reductions and treatment discontinuations,” says Dr Rocío García-Carbonero from Hospital Universitario 12 de Octubre, Madrid, Spain. Selpercatinib is already approved for MTC based on high response rates in MKI-naïve patients and after MKI failure in the LIBRETTO-001 basket trial (N Engl J Med. 2020;383:825–835), which was non-controlled and non-randomised. “The LIBRETTO-531 trial includes a large number of patients considering this is not a common disease. It is now unquestionable that selpercatinib should be the first-line choice for RET-mutated MTC, although I believe many centres are already using it first-line for patients known to be RET-positive.”
García-Carbonero stresses the importance of testing all patients for RET mutations, which are very common in MTC and are present in >95% of hereditary carcinomas and around half of sporadic carcinomas (iScience. 2019;20:324–336). “The problem is that RET testing is not routinely performed in all centres and this needs to be addressed,” she continues. “RET testing is generally performed on blood samples after family genetic counselling, but for non-hereditary cases it may only be assessed in larger centres. Upfront testing should be routine for all patients before starting therapy so that those with RET mutations can receive selpercatinib.”
Another presentation in Madrid showed that the phase II O6-methylguanine-DNA methyltransferase-NET (MGMT-NET) trial did not report an objective response rate (ORR) improvement at 3 months with alkylating agents compared with oxaliplatin in patients with advanced pancreatic, thoracic or unknown primary NETs (LBA54). However, the best ORR data suggested that alkylating agents (18/34, 52.9%) provided a higher ORR than oxaliplatin (6/24, 25.0%) in patients with deficient MGMT, whereas ORR appeared higher with oxaliplatin (7/18, 38.9%) than with alkylating agents (3/26, 11.5%) in patients with proficient MGMT. This was not clearly associated, however, with differences in PFS or OS.
García-Carbonero says, “This is also an important investigator-initiated trial, but I believe the study is probably underpowered to answer the question. Patients were stratified by proficient versus deficient MGMT status before being randomised to treatment groups, which is an appropriate design aiming to address a clinically relevant question; however, patient numbers per group were small and comprised mixed populations, including different tumour grades and primary tumour sites, which affects prognosis and may complicate interpretation of trial outcomes. The results of the MGMT-NET trial may be consistent with its hypothesis in terms of ORR, although no clear impact on long-term outcomes was demonstrated with this strategy. The hypothesis that MGMT status may predict response to alkylating agents is biologically plausible and has been demonstrated in other tumour types, but larger studies would be required for definitive confirmation in NETs.”
Regarding predictive markers, García-Carbonero advises, “At present, other than somatostatin receptors, there are no validated predictive markers to use for targeted therapy for NETs or to predict responses to treatment. These tumours do not have many recurrent mutations and so patients generally receive several treatment options, but in different sequences in the hope that one is efficacious. There is much further work to be done to help guide personalised medicine and expand the treatment options for patients with NETs.”
Abstracts discussed:
Hadoux J, et al. Randomized phase 3 study of selpercatinib versus cabozantinib or vandetanib in advanced, kinase inhibitor-naïve, RET-mutant medullary thyroid cancer. ESMO Congress 2023, LBA3
Presidential Symposium 1, 21.10.2023, h. 16:30 – 18:15, Madrid Auditorium – Hall 6
Walter T, et al. Alkylating agent-based vs oxaliplatin-based chemotherapy in neuroendocrine tumours according to the O6-methylguanine-DNA methyltransferase (MGMT) status: a randomized phase II study (MGMT-NET) on behalf of the French Group of Endocrine Tumors (GTE) and ENDOCAN-RENATEN network. ESMO Congress 2023, LBA54
Proffered Paper Session – NETs and endocrine tumours, 22.10.2023, h. 08:30 – 10:00, Toledo Auditorium – Hall 3