The fields of metastatic pancreatic NETs and advanced and low proliferative grade 3 disease tumours move forward
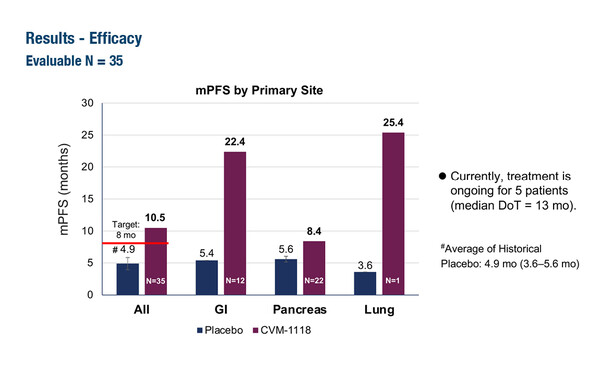
Data presented at the ESMO Congress 2024 (Barcelona, 13–17 September) demonstrated the potential of three novel antiangiogenic agents for the treatment of neuroendocrine tumours (NETs). Results from a phase IIa, single-arm study showed a superior tolerability and safety profile of an oral anti-vasculogenic mimicry agent, CVM-1118, compared with current targeted therapies in 43 patients with advanced NETs who had progressed on prior standard of care (Abstract 1145MO). With a median follow up of 12.8 months and, on average, three prior therapies, patients achieved median progression-free survival (PFS) of 10.5 months (95% confidence interval [CI] 5.6–22.4 months), which was longer than the 4–6 months for placebo groups reported in phase III trials of sunitinib (N Engl J Med. 2011;364:501–513) and everolimus (N Engl J Med. 2011;364:514–523). A subgroup analysis of CVM-1118 with concurrent somatostatin analogue showed a median PFS of 22.4 months.
Commenting on the study results, Dr Louis de Mestier from the University Paris-Cité, France, says, “The results must be interpreted with caution as patients with early progression in the study could have received concurrent somatostatin analogues, so the PFS of over 2 years in the combination treatment subgroup may have positively influenced the PFS of the whole cohort. However, these results clearly warrant further exploration in controlled clinical trials.”
In a second phase II study, RamuNET, which assessed a combination of an antiangiogenic and alkylating agent, ramucirumab and dacarbazine, results showed it to be a promising new treatment option in patients with progressive, well-differentiated, pre-treated metastatic pancreatic NETs (grades1–3) (Abstract 1147MO). Out of a total of 45 patients, the disease control rate (DCR) after 6 months was 71.1%. During the first 6 months, nine patients had progressive disease and four terminated treatment early due to adverse events. “There are already some signals that this type of combination is a promising strategy in patients with advanced pancreatic NETs, for example streptozotocin plus bevacizumab (Eur J Cancer. 2014;50:3098–3106),” says de Mestier commenting on the data presented. “However, as there was no control arm in the study, it is difficult to ascertain whether the combination could lead to an additional or synergistic effect.”
The uncertain significance of outcomes from an interim analysis of the CABONEN prospective, single-arm, multicentre phase II trial, which was also presented at the Congress, are highlighted by de Mestier (Abstract 1146MO). The study investigated the efficacy and safety of cabozantinib in 32 patients with advanced, low proliferative grade 3 NETs with Ki67 up to 60%. “The DCR after 6 months was 64.1%, and the median PFS was 6.7 months, which seems rather low in this population, possibly because the study included some patients with neuroendocrine carcinomas and 71% of patients were pre-treated,” he says. “Although studies and populations are not comparable, subgroup analyses of the NETTER-2 trial showed median PFS of 22.2 and 5.6 months in patients with treatment-naïve advanced grade 3 NETs treated with 177Lu-DOTA-TATE plus octreotide or double-dose octreotide, respectively (Lancet. 2024;403:2807–2817).” Also, a total of 37 serious adverse events (SAEs) were observed, including three grade 5 events, highlighting that, “tolerance to cabozantinib can be a problem.”
Regarding the design of these studies, de Mestier adds: “All the studies that have been presented used the RECIST criteria to determine response, as is usually done; however, these criteria are not suitable for some antiangiogenic agents, as previously shown with sunitinib, and further criteria are required for clinical trials and routine practice.”
The ability to predict the efficacy of agents is one of the most important gaps in relation to antiangiogenic-based therapy for NETs, and predictive biomarkers are crucial.
He concludes: “To close current knowledge gaps, we need both plasma and imaging biomarkers, as well as reproducible and robust plasma assays for routine clinical practice. We also require comparative and strategy studies to identify which agents should be used and in which order, especially if resistance develops.”
Programme details
Yen CJ, et al. CVM-005: Phase IIa study of CVM-1118, a novel oral anti-vasculogenic mimicry (VM) agent, in advanced neuroendocrine tumors (NET) after progression on prior therapy. ESMO Congress 2024, Abstract 1145MO
Mini Oral Session – NETs and endocrine tumours, 13.09.2024, h. 16:00 – 17:30, Pamplona Auditorium – Hall 3
Koenig AO, et al. Interim analysis of CABONEN – a multicenter phase II trial investigating cabozantinib in patients with advanced, low proliferative NEN G3. ESMO Congress 2024, Abstract 1146MO
Mini Oral Session – NETs and endocrine tumours, 13.09.2024, h. 16:00 – 17:30, Pamplona Auditorium – Hall 3
Krug S, et al. Ramucirumab in combination with dacarbazine in patients with progressive well-differentiated metastatic pancreatic neuroendocrine tumors (RamuNET): An AIO phase II multicenter single-arm study. ESMO Congress 2024, Abstract 1147MO
Mini Oral Session – NETs and endocrine tumours, 13.09.2024, h. 16:00 – 17:30, Pamplona Auditorium – Hall 3