Positive findings from the DESTINY-Breast05 and DESTINY-Breast11 trials suggest that a paradigm change in the treatment of high-risk patients may be imminent
Following demonstration that novel antibody–drug conjugates (ADCs) are highly efficacious with reduced toxicity compared with conventional chemotherapy in the metastatic setting across a broad range of malignancies (Exp Hematol Oncol. 2025; 14:41), thus becoming standard of care (SOC) in many countries for patients with advanced breast cancer, this selective class of anticancer agents is now moving to the curative setting in patients with early HER2-positive breast cancer.
Novel insights into the use of neoadjuvant ADCs were provided by interim data from the DESTINY-Breast05 trial presented during today’s Presidential Symposium at the ESMO Congress 2025 (Berlin, 17–21 October), which showed a statistically significant clinical benefit with trastuzumab deruxtecan (T-DXd) over trastuzumab emtansine (T-DM1) (LBA1, key results in the box below). T-DM1 is the only ADC currently approved for patients with residual invasive disease following neoadjuvant therapy, after demonstrating a 50% reduction in risk of recurrence or death compared with trastuzumab (N Engl J Med. 2019;380:617–628).
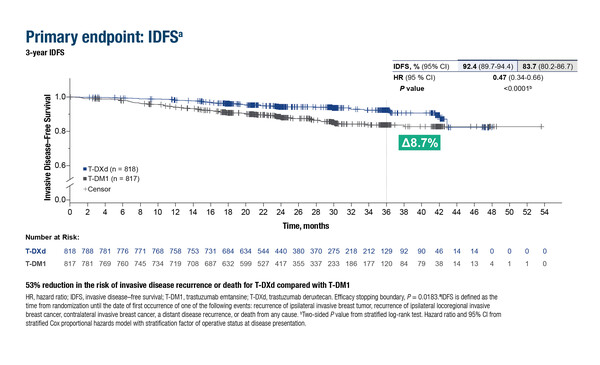
As reported in Berlin, in 1,635 patients with residual, invasive HER2-positive breast cancer at high risk for recurrence after neoadjuvant treatment with taxane-based chemotherapy and anti-HER2 therapy, T-DXd improved invasive disease-free survival (IDFS) – the primary endpoint – and disease-free survival (DFS) by 53% compared with T-DM1 (for both: hazard ratio [HR] 0.47; 95% confidence interval [CI] 0.34–0.66; p<0.0001).
Explaining the rationale, Dr Evandro de Azambuja from the Jules Bordet Institute, Brussels, Belgium, notes, “Patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant therapy are at a high risk of recurrence, so there is a particular need for therapies to ensure patients achieve pathological complete response (pCR) following neoadjuvant therapies, and a high unmet need to treat residual disease in those who do not achieve pCR to prevent the development of metastasis.” Data presented also demonstrated a clinically meaningful improvement in brain metastasis-free interval (BMFI) with T-DXd versus T-DM1 (HR 0.64; 95% CI 0.35–1.17). “This is extremely important because brain metastases are associated with a dismal prognosis. We know that T-DXd has much more activity in the brain than T-DM1 and the data confirm this,” adds de Azambuja.
In the DESTINY-Breast05, similar rates of treatment-emergent adverse events (AEs) were reported in the treatment arms (grade ≥3 in 50.6% and 51.9% of patients treated with T-DXd and T-DM1, respectively), and there were very few deaths. However, more adjudicated drug-related interstitial lung disease was seen with T-DXd than T-DM1 (9.6% [2 patients with grade 5] versus 1.6% [0 patients with grade 5]). “This is also found in the metastatic setting and suggests that patients should be monitored with regular scans if using T-DXd in early breast cancer,” cautions de Azambuja. “However, the generally manageable safety profile and the superior efficacy data suggest that T-DXd should replace T-DM1 as the new SOC for patients with HER2-positive, residual invasive breast cancer after neoadjuvant therapy.”
The Presidential Symposium also saw further positive data being presented, from the DESTINY-Breast11 study, in which neoadjuvant T-DXd (n=321) and dose-dense doxorubicin plus cyclophosphamide (ddAC; n=320), each followed by paclitaxel, trastuzumab plus pertuzumab (THP), led to pCR rates of 67.3% and 56.3%, respectively (difference in pCR rate 11.2%; 95% CI 4.0–18.3; p=0.003) in untreated patients with high-risk HER-positive early breast cancer (Abstract 291O, key results in the box below). The difference in favour of T-DXd-THP was regardless of hormone receptor status, with pCR rates of 61.4% versus 52.3%, respectively, in hormone receptor-positive disease and 83.1% versus 67.1%, respectively, in hormone receptor-negative disease. “This is interesting because with existing SOC regimens, pCR tends to be lower in patients with hormone receptor-positive than hormone receptor-negative disease,” notes de Azambuja. An early trend towards a benefit with T-DXd-THP in event-free survival was also reported at the data cutoff with a median duration of follow-up of ~24 months in both treatment arms (HR 0.56; 95% CI 0.26–1.17), suggesting that patients are living longer without events indicative of relapse.
Grade ≥3 AEs were reported in 37.5% of patients in the T-DXd-THP arm compared with 55.8% in the ddAC-THP arm, with lower frequency of any-grade left ventricular dysfunction with T-DXd-THP (1.3% versus 6.1%). “The T-DXd regimen has the added advantage of an improved safety profile compared with the anthracycline-containing regimen,” comments de Azambuja. “We find that some patients treated with the T-DXd regimen can experience prolonged nausea, but this ican be generally managed and mitigated, and apart from that, the drug is well tolerated.” Grade ≥3 nausea was reported in 1.9% of patients in the T-DXd-THP arm compared with 0.3% in the ddAC-THP arm. Taken together, these extremely positive results position T-DXd-THP as a potential new neoadjuvant therapy for high-risk HER2-positive early breast cancer, although additional survival data are needed.
De Azambuja concludes: “We are witnessing a changing treatment paradigm for patients with HER2-positive early breast cancer. The studies with T-DXd are very positive and will translate into cure for more of our high-risk patients. We will be able to improve prognosis, either by preventing the development of metastatic disease by achieving pCR, or by treating residual disease and reducing the risk of disease progression and brain metastasis.”
Programme details:
Geyer C, et al. Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (pts) with high-risk human epidermal growth factor receptor 2–positive (HER2+) primary breast cancer (BC) with residual invasive disease after neoadjuvant therapy (tx): Interim analysis of DESTINY-Breast05. ESMO Congress 2025 - LBA1
- N=1,635 (n=818 T-DXd; n=817 T-DM1)
- For T-DXd vs T-DM1:
- IDFS: 92.4% vs 83.7% (51 events vs 102 events); HR 0.47; p<0.0001
- DFS: 92.3% vs 83.5% (HR 0.47; p<0.0001)
- BMFI: 97.6% vs 95.8%; HR 0.64
- Grade ≥3 TEAEs: 50.6% vs 51.9%
- Adjudicated drug-related ILD: 9.6% (2 grade 5) vs 1.6% (0 grade 5)
Harbeck N, et al. DESTINY-Breast11: neoadjuvant trastuzumab deruxtecan alone (T-DXd) or followed by paclitaxel + trastuzumab + pertuzumab (T-DXd-THP) vs SOC for high-risk HER2+ early breast cancer (eBC). ESMO Congress 2025 - Abstract 291O
- N=927 (n=321 T-DXd-THP; n=320 ddAC-THP; n=286 T-DXd)
- For T-DXd-THP vs ddAC-THP:
- pCR: 67.3% vs 56.3% (ΔpCR rate 11.2%; p=0.003)
- Hormone receptor+: 61.4% vs 52.3% (ΔpCR rate 9.1%)
- Hormone receptor−: 83.1% vs 67.1% (ΔpCR rate 16.1%)
- Grade ≥3 AEs: 37.5% vs 55.8%
- Any SAE: 10.6% vs 20.2%
- Left ventricular dysfunction: 1.3% vs 6.1%
- pCR: 67.3% vs 56.3% (ΔpCR rate 11.2%; p=0.003)